Omadacycline Pharmacokinetics: Influence of Mortality Risk Score among Patients with Community-Acquired Bacterial Pneumonia
- PMID: 36533934
- PMCID: PMC9872632
- DOI: 10.1128/aac.02201-21
Omadacycline Pharmacokinetics: Influence of Mortality Risk Score among Patients with Community-Acquired Bacterial Pneumonia
Keywords: community-acquired bacterial pneumonia; omadacycline; pharmacokinetics.
Conflict of interest statement
The authors declare a conflict of interest. M.T., M.C.S., J.P.H., S.M.B., and C.M.R. are employees of the Institute for Clinical Pharmacodynamics, Inc., which has received research support from Paratek Pharmaceuticals, Inc. E.A.L. was an employee of ICPD at the time the analyses were performed and is now and employee of Vertex Pharmaceuticals, Inc. L.F. was an employee of Paratek Pharmaceuticals, Inc., at the time the analyses were performed and is now an employee of AN2 Therapeutics. J.N.S. was an employee of Paratek Pharmaceuticals, Inc., at the time the analyses were performed and is now an employee of Scientific and Medical Affairs Consulting, LLC. P.C.M. was an employee of Paratek Pharmaceuticals, Inc., at the time the analyses were performed and is now an employee of VenatoRx, Inc., and E.T. was an employee of Paratek Pharmaceuticals, Inc., at the time the analyses were performed and is now an employee of Neuraptive Therapeutics, Inc.
Figures
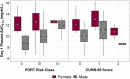
References
-
- Paratek Pharmaceuticals, Inc. 2021. Nuzyra (omadacycline) package insert. Paratek Pharmaceuticals, Inc., Boston, MA.
-
- Paratek Pharmaceuticals. 2018. Omadacycline p-toluenesulfonate tablets and injection for the treatment of acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP). Briefing document for the Antimicrobial Drugs Advisory Committee (AMDAC). https://www.fda.gov/media/115100/download. Accessed 26 September 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

