Serum Neurofilament Light Trajectories and Their Relation to Subclinical Radiological Disease Activity in Relapsing Multiple Sclerosis Patients in the APLIOS Trial
- PMID: 36534274
- PMCID: PMC9837345
- DOI: 10.1007/s40120-022-00427-8
Serum Neurofilament Light Trajectories and Their Relation to Subclinical Radiological Disease Activity in Relapsing Multiple Sclerosis Patients in the APLIOS Trial
Abstract
Introduction: Several studies have described prognostic value of serum neurofilament light chain (sNfL) at the group level in relapsing multiple sclerosis (RMS) patients. Here, we aimed to explore the temporal association between sNfL and development of subclinical disease activity as assessed by magnetic resonance imaging (MRI) at the group level and evaluate the potential of sNfL as a biomarker for capturing subclinical disease activity in individual RMS patients.
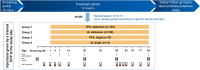
Methods: In the 12-week APLIOS study, patients (N = 284) received subcutaneous ofatumumab 20 mg. Frequent sNfL sampling (14 time points over 12 weeks) and monthly MRI scans enabled key analyses including assessment of the group-level temporal relationship of sNfL levels with on-study subclinical development of gadolinium-enhancing (Gd +)T1 lesions. Prognostic value of baseline sNfL ("high" vs. "low") level for subsequent on-study clinical relapse or Gd + T1 activity was assessed. Individual patient-level development of on-study Gd + T1 lesions was compared across three predictors: baseline Gd + T1 lesion number, baseline sNfL ("high" vs. "low"), and time-matched sNfL.
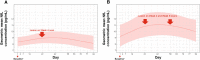
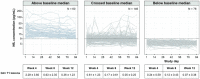
Results: In patients developing Gd + T1 lesions at week 4 (absent at baseline), sNfL levels increased during the month preceding the week-4 MRI scan and then gradually decreased back to baseline. High versus low baseline sNfL conferred increased risk of subsequent on-study clinical relapse or Gd + T1 activity (HR, 2.81; p < 0.0001) in the overall population and, notably, also in the patients without baseline Gd + T1 lesions (HR, 2.48; p = 0.0213). Individual patient trajectories revealed a marked difference in Gd + T1 lesions between patients with the ten highest vs. lowest baseline sNfL levels (119 vs. 19 lesions). Prognostic value of baseline or time-matched sNfL for on-study Gd + T1 lesions was comparable to that of the number of baseline MRI Gd + T1 lesions.
Conclusions: sNfL measurement may have utility in capturing and monitoring subclinical disease activity in RMS patients. sNfL assessments could complement regular MRI scans and may provide an alternative when MRI assessment is not feasible.
Clinicaltrials: GOV: NCT03560739.
Classification of evidence: This study provides class I evidence that serum neurofilament light may be used as a biomarker for monitoring subclinical disease activity in relapsing multiple sclerosis patients, as shown by its elevation in the weeks preceding the development of new gadolinium-enhancing T1 lesion activity.
Keywords: Biomarker; Ofatumumab; Relapsing multiple sclerosis; Serum neurofilament light chain; Subclinical disease activity.
© 2022. The Author(s).
Figures




References
-
- Joy JE, Johnston RB Jr, editors. Committee on multiple sclerosis: current status and strategies for the future, board on neuroscience and behavioral health. Washington: National Academies Press (US); 2001. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

