Real-world patient characteristics and use of disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis: a cross-national study
- PMID: 36534353
- PMCID: PMC10017582
- DOI: 10.1007/s10067-022-06478-4
Real-world patient characteristics and use of disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis: a cross-national study
Abstract
Introduction: Rheumatoid arthritis (RA) is associated with significant morbidity and economic burden. This study aimed to compare baseline characteristics and patterns of anti-inflammatory drug use and disease-modifying anti-rheumatic drug (DMARD) use among patients with RA in Southern Italy versus the United States.
Method: Using Caserta Local Health Unit (Italy) and Optum's de-identified Clinformatics® Data Mart (United States) claims databases, patients with ≥ 2 diagnosis codes for RA during the study period (Caserta: 2010-2018; Optum: 2010-2019) were identified. Baseline patient characteristics, as well as proportion of RA patients untreated/treated with NSAIDs/glucocorticoids/conventional DMARDs (csDMARDs)/biological/targeted synthetic DMARDs (b/tsDMARDs) during the first year of follow-up, and the proportion of RA patients with ≥ 1 switch/add-on between the first and the second year of follow-up, were calculated. These analyses were then stratified by age group (< 65; ≥ 65).
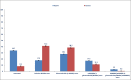
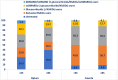
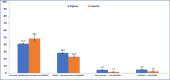
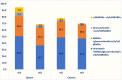
Results: A total of 9227 RA patients from Caserta and 195,951 from Optum databases were identified (two-thirds were females). During the first year of follow-up, 45.9% RA patients from Optum versus 79.9% from Caserta were exclusively treated with NSAIDs/glucocorticoids; 17.2% versus 11.3% from Optum and Caserta, respectively, were treated with csDMARDs, mostly methotrexate or hydroxychloroquine in both cohorts. Compared to 0.6% of RA patients from Caserta, 3.2% of the Optum cohort received ≥ 1 b/tsDMARD dispensing. Moreover, 61,655 (33.7%) patients from Optum cohort remained untreated compared to 748 (8.3%) patients from the Caserta cohort. The subgroup analyses stratified by age showed that 42,989 (39.8%) of elderly RA patients were untreated compared to 18,666 (24.9%) young adult RA patients in Optum during the first year of follow-up. Moreover, a higher proportion of young adult RA patients was treated with b/tsDMARDs, with and without csDMARDs, compared to elderly RA patients (Optum<65: 6.4%; Optum≥65: 1.0%; P-value < 0.001; Caserta<65: 0.8%; Caserta≥65: 0.1%; P-value < 0.001). Among RA patients untreated during the first year after ID, 41.2% and 48.4% RA patients from Caserta and Optum, respectively, received NSAIDs, glucocorticoids, and cs/b/tsDMARDs within the second year of follow-up. Stratifying the analysis by age groups, 50.6% of untreated young RA patients received study drug dispensing within the second year of follow-up, compared to only 36.7% of elderly RA patients in Optum. Interestingly, more young adult RA patients treated with csDMARDs during the first year after ID received a therapy escalation to b/tsDMARD within the second year after ID in both cohorts, compared to elderly RA patients (Optum<65: 7.8%; Optum≥65: 1.8%; Caserta<65: 3.2%; Caserta≥65: 0.6%).
Conclusions: Most of RA patients, with heterogeneous baseline characteristics in Optum and Caserta cohorts, were treated with anti-inflammatory/csDMARDs rather than bDMARDs/tsDMARDs during the first year post-diagnosis, especially in elderly RA patients, suggesting a need for better understanding and dealing with barriers in the use of these agents for RA patients. Key Points • Substantial heterogeneity in baseline characteristics and access to bDMARD or tsDMARD drugs between RA patients from the United States and Italy exists. • Most of RA patients seem to be treated with anti-inflammatory/csDMARD drugs rather than bDMARD/tsDMARD drugs during the first year post-diagnosis. • RA treatment escalation is less frequent in old RA patients than in young adult RA patients. • An appropriate use of DMARDs should be considered to achieve RA disease remission or low disease activity.
Keywords: Biologics; Claims database; Disease-modifying anti-rheumatic drugs; Real-world data; Rheumatoid arthritis.
© 2022. The Author(s).
Conflict of interest statement
Ylenia Ingrasciotta is the CEO of the academic spin-off “INSPIRE srl” of the University of Messina, which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.) and from pharmaceutical Companies (Chiesi Italia, Kyowa Kirin s.r.l., Daiichi Sankyo Italia S.p.A.). Gianluca Trifirò has served on advisory boards/seminars funded by SANOFI, Eli Lilly, AstraZeneca, Abbvie, Servier, Mylan, Gilead, and Amgen; he was the scientific director of a II level Master on pharmacovigilance, pharmacoepidemiology, and real-world evidence, which has received non-conditional contributions from various pharmaceutical companies; he coordinates a pharmacoepidemiology team at the University of Messina, which has received funding for conducting observational studies from various pharmaceutical companies (Boehringer Ingelheim, Daiichi Sankyo, PTC Pharmaceuticals). He is also chief of the academic spin-off “INSPIRE srl,” which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.) from pharmaceutical Companies (Chiesi Italia, Kyowa Kirin s.r.l., Daiichi Sankyo Italia S.p.A.). Seoyoung C. Kim has received research grants to the Brigham and Women’s Hospital from Pfizer, Roche, AbbVie, and Bristol-Myers Squibb for unrelated studies. She is also supported by NIH-K24AR078959. The other authors declare that they have no conflicts of interest.
Figures
References
-
- Erol K, Gok K, Cengiz G, Kilic G, Kilic E, Ozgocmen S. Extra-articular manifestations and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis. Acta Reumatol Port. 2018;43(1):32–39. - PubMed
-
- World Health Organization (2021) Chronic rheumatic conditions. Available online: https://www.who.int/chp/topics/rheumatic/en/#:~:text=Rheumatic%20or%20mu.... Accessed on 10 May 2021
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

