Association of Early Progression Independent of Relapse Activity With Long-term Disability After a First Demyelinating Event in Multiple Sclerosis
- PMID: 36534392
- PMCID: PMC9856884
- DOI: 10.1001/jamaneurol.2022.4655
Association of Early Progression Independent of Relapse Activity With Long-term Disability After a First Demyelinating Event in Multiple Sclerosis
Abstract
Importance: Progression independent of relapse activity (PIRA) is the main event responsible for irreversible disability accumulation in relapsing multiple sclerosis (MS).
Objective: To investigate clinical and neuroimaging predictors of PIRA at the time of the first demyelinating attack and factors associated with long-term clinical outcomes of people who present with PIRA.
Design, setting, and participants: This cohort study, conducted from January 1, 1994, to July 31, 2021, included patients with a first demyelinating attack from multiple sclerosis; patients were recruited from 1 study center in Spain. Patients were excluded if they refused to participate, had alternative diagnoses, did not meet protocol requirements, had inconsistent demographic information, or had less than 3 clinical assessments.
Exposures: Exposures included (1) clinical and neuroimaging features at the first demyelinating attack and (2) presenting PIRA, ie, confirmed disability accumulation (CDA) in a free-relapse period at any time after symptom onset, within (vs after) the first 5 years of the disease (ie, early/late PIRA), and in the presence (vs absence) of new T2 lesions in the previous 2 years (ie, active/nonactive PIRA).
Main outcomes and measures: Expanded Disability Status Scale (EDSS) yearly increase rates since the first attack and adjusted hazard ratios (HRs) for predictors of time to PIRA and time to EDSS 6.0.
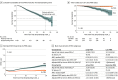
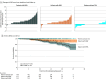
Results: Of the 1128 patients (mean [SD] age, 32.1 [8.3] years; 781 female individuals [69.2%]) included in the study, 277 (25%) developed 1 or more PIRA events at a median (IQR) follow-up time of 7.2 (4.6-12.4) years (for first PIRA). Of all patients with PIRA, 86 of 277 (31%) developed early PIRA, and 73 of 144 (51%) developed active PIRA. Patients with PIRA were slightly older, had more brain lesions, and were more likely to have oligoclonal bands than those without PIRA. Older age at the first attack was the only predictor of PIRA (HR, 1.43; 95% CI, 1.23-1.65; P < .001 for each older decade). Patients with PIRA had steeper EDSS yearly increase rates (0.18; 95% CI, 0.16-0.20 vs 0.04; 95% CI, 0.02-0.05; P < .001) and an 8-fold greater risk of reaching EDSS 6.0 (HR, 7.93; 95% CI, 2.25-27.96; P = .001) than those without PIRA. Early PIRA had steeper EDSS yearly increase rates than late PIRA (0.31; 95% CI, 0.26-0.35 vs 0.13; 95% CI, 0.10-0.16; P < .001) and a 26-fold greater risk of reaching EDSS 6.0 from the first attack (HR, 26.21; 95% CI, 2.26-303.95; P = .009).
Conclusions and relevance: Results of this cohort study suggest that for patients with multiple sclerosis, presenting with PIRA after a first demyelinating event was not uncommon and suggests an unfavorable long-term prognosis, especially if it occurs early in the disease course.
Conflict of interest statement
Figures

Comment in
-
Time to Change the Current Clinical Classification of Multiple Sclerosis?JAMA Neurol. 2023 Feb 1;80(2):128-130. doi: 10.1001/jamaneurol.2022.4156. JAMA Neurol. 2023. PMID: 36534379 No abstract available.
References
-
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636. https://linkinghub.elsevier.com/retrieve/pii/S0140673618304811. doi:10.1016/S0140-6736(18)30481-1 - DOI - PubMed
-
- Kappos L, Wolinsky JS, Giovannoni G, et al. . Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132-1140. doi:10.1001/jamaneurol.2020.1568 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

