Adult type diffuse gliomas in the new 2021 WHO Classification
- PMID: 36534419
- PMCID: PMC9763975
- DOI: 10.32074/1591-951X-823
Adult type diffuse gliomas in the new 2021 WHO Classification
Abstract
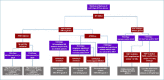
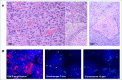
Adult-type diffuse gliomas represent a group of highly infiltrative central nervous system tumors with a prognosis that significantly varies depending on the specific subtype and histological grade. Traditionally, adult-type diffuse gliomas have been classified based on their morphological features with a great interobserver variability and discrepancy in patient survival even within the same histological grade. Over the last few decades, advances in molecular profiling have drastically changed the diagnostic approach and classification of brain tumors leading to the development of an integrated morphological and molecular classification endowed with a more clinically relevant value. These concepts were largely anticipated in the revised fourth-edition of WHO classification of central nervous system tumors published in 2016. The fifth-edition (WHO 2021) moved molecular diagnostics forward into a full integration of molecular parameters with the histological features into an integrative diagnostic approach. Diagnosis of adult type diffuse gliomas, IDH mutant and IDH-wildtype has been simplified by introducing revised diagnostic and grading criteria. In this review, we will discuss the most recent updates to the classification of adult-type diffuse gliomas and summarize the essential diagnostic keys providing a practical guidance to pathologists.
Keywords: IDH mutant; IDH-WT; astrocytoma; glioblastoma.
Copyright © 2022 Società Italiana di Anatomia Patologica e Citopatologia Diagnostica, Divisione Italiana della International Academy of Pathology.
Figures


References
-
- Ostrom QT, Cioffi G, Waite K, et al. . CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol 2021;23(12 Suppl 2): iii1-iii105. https://doi.org/10.1093/neuonc/noab200 10.1093/neuonc/noab200 - DOI - PMC - PubMed
-
- Labussière M, Boisselier B, Mokhtari K, et al. . Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 2014;83:1200-1206. https://doi.org/10.1212/WNL.0000000000000814 10.1212/WNL.0000000000000814 - DOI - PubMed
-
- Cahill DP, Louis DN, Cairncross JG: Molecular background of oligodendroglioma: 1p/19q, IDH, TERT, CIC and FUBP1. CNS Oncol 2015;4:287-294. https://doi.org/10.2217/cns.15.32 10.2217/cns.15.32 - DOI - PMC - PubMed
-
- Brat DJ, Aldape K, Colman H, et al. . cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol 2020;139:603-608. https://doi.org/10.1007/s00401-020-02127-9 10.1007/s00401-020-02127-9 - DOI - PMC - PubMed
-
- Brat DJ, Aldape K, Colman H, et al. . cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol 2018;136:805-810. https://doi.org/10.1007/s00401-018-1913-0 10.1007/s00401-018-1913-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

