Comprehensive characterization of toxins during progression of inhalation anthrax in a non-human primate model
- PMID: 36534695
- PMCID: PMC9810172
- DOI: 10.1371/journal.ppat.1010735
Comprehensive characterization of toxins during progression of inhalation anthrax in a non-human primate model
Abstract
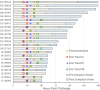
Inhalation anthrax has three clinical stages: early-prodromal, intermediate-progressive, and late-fulminant. We report the comprehensive characterization of anthrax toxins, including total protective antigen (PA), total lethal factor (LF), total edema factor (EF), and their toxin complexes, lethal toxin and edema toxin in plasma, during the course of inhalation anthrax in 23 cynomolgus macaques. The toxin kinetics were predominantly triphasic with an early rise (phase-1), a plateau/decline (phase-2), and a final rapid rise (phase-3). Eleven animals had shorter survival times, mean±standard deviation of 58.7±7.6 hours (fast progression), 11 animals had longer survival times, 113±34.4 hours (slow progression), and one animal survived. Median (lower-upper quartile) LF levels at the end-of-phase-1 were significantly higher in animals with fast progression [138 (54.9-326) ng/mL], than in those with slow progression [23.8 (15.6-26.3) ng/mL] (p = 0.0002), and the survivor (11.1 ng/mL). The differences were also observed for other toxins and bacteremia. Animals with slow progression had an extended phase-2 plateau, with low variability of LF levels across all time points and animals. Characterization of phase-2 toxin levels defined upper thresholds; critical levels for exiting phase-2 and entering the critical phase-3, 342 ng/mL (PA), 35.8 ng/mL (LF), and 1.10 ng/mL (EF). The thresholds were exceeded earlier in animals with fast progression (38.5±7.4 hours) and later in animals with slow progression (78.7±15.2 hours). Once the threshold was passed, toxin levels rose rapidly in both groups to the terminal stage. The time from threshold to terminal was rapid and similar; 20.8±7.4 hours for fast and 19.9±7.5 hours for slow progression. The three toxemic phases were aligned with the three clinical stages of anthrax for fast and slow progression which showed that anthrax progression is toxin- rather than time-dependent. This first comprehensive evaluation of anthrax toxins provides new insights into disease progression.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Helgason E, Okstad OA, Caugant DA, Johansen HA, Fouet A, Mock M, et al. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl Environ Microbiol. 2000;66(6):2627–30. doi: 10.1128/AEM.66.6.2627-2630.2000 ; PubMed Central PMCID: PMC110590. - DOI - PMC - PubMed
-
- Friedlander AM. Anthrax. In: Zajtchuk R, editor. Textbook of Military Medicine: Medical Aspects of Chemical and Biological Warfare. 5. Washington, D.C.: Office of The Surgeon General; 1997. p. 467–78.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

