Population heterogeneity in Plasmodium vivax relapse risk
- PMID: 36534705
- PMCID: PMC9810152
- DOI: 10.1371/journal.pntd.0010990
Population heterogeneity in Plasmodium vivax relapse risk
Abstract
A key characteristic of Plasmodium vivax parasites is their ability to adopt a latent liver-stage form called hypnozoites, able to cause relapse of infection months or years after a primary infection. Relapses of infection through hypnozoite activation are a major contributor to blood-stage infections in P vivax endemic regions and are thought to be influenced by factors such as febrile infections which may cause temporary changes in hypnozoite activation leading to 'temporal heterogeneity' in reactivation risk. In addition, immunity and variation in exposure to infection may be longer-term characteristics of individuals that lead to 'population heterogeneity' in hypnozoite activation. We analyze data on risk of P vivax in two previously published data sets from Papua New Guinea and the Thailand-Myanmar border region. Modeling different mechanisms of reactivation risk, we find strong evidence for population heterogeneity, with 30% of patients having almost 70% of all P vivax infections. Model fitting and data analysis indicates that individual variation in relapse risk is a primary source of heterogeneity of P vivax risk of recurrences. Trial Registration: ClinicalTrials.gov NCT01640574, NCT01074905, NCT02143934.
Copyright: © 2022 Stadler et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

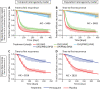


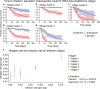
References
-
- World Health Organization. World Malaria Report 2021. 2021.
-
- Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CSN, et al.. Strategies for Understanding and Reducing the Plasmodium vivax and Plasmodium ovale Hypnozoite Reservoir in Papua New Guinean Children: A Randomised Placebo-Controlled Trial and Mathematical Model. PLoS Med. 2015;12(10):e1001891. doi: 10.1371/journal.pmed.1001891 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

