Collective Diffraction Effects in Perovskite Nanocrystal Superlattices
- PMID: 36534898
- PMCID: PMC9813911
- DOI: 10.1021/acs.accounts.2c00613
Collective Diffraction Effects in Perovskite Nanocrystal Superlattices
Abstract
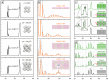
ConspectusFor almost a decade now, lead halide perovskite nanocrystals have been the subject of a steadily growing number of publications, most of them regarding CsPbBr3 nanocubes. Many of these works report X-ray diffraction patterns where the first Bragg peak has an unusual shape, as if it was composed of two or more overlapping peaks. However, these peaks are too narrow to stem from a nanoparticle, and the perovskite crystal structure does not account for their formation. What is the origin of such an unusual profile, and why has it been overlooked so far? Our attempts to answer these questions led us to revisit an intriguing collective diffraction phenomenon, known for multilayer epitaxial thin films but not reported for colloidal nanocrystals before. By analogy, we call it the multilayer diffraction effect.Multilayer diffraction can be observed when a diffraction experiment is performed on nanocrystals packed with a periodic arrangement. Owing to the periodicity of the packing, the X-rays scattered by each particle interfere with those diffracted by its neighbors, creating fringes of constructive interference. Since the interfering radiation comes from nanoparticles, fringes are visible only where the particles themselves produce a signal in their diffraction pattern: for nanocrystals, this means at their Bragg peaks. Being a collective interference phenomenon, multilayer diffraction is strongly affected by the degree of order in the nanocrystal aggregate. For it to be observed, the majority of nanocrystals within the sample must abide to the stacking periodicity with minimal misplacements, a condition that is typically satisfied in self-assembled nanocrystal superlattices or stacks of colloidal nanoplatelets.A qualitative understanding of multilayer diffraction might explain why the first Bragg peak of CsPbBr3 nanocubes sometimes appears split, but leaves many other questions unanswered. For example, why is the split observed only at the first Bragg peak but not at the second? Why is it observed routinely in a variety of CsPbBr3 nanocrystals samples and not just in highly ordered superlattices? How does the morphology of particles (i.e., nanocrystals vs nanoplatelets) affect the appearance of multilayer diffraction effects? Finally, why is multilayer diffraction not observed in other popular nanocrystals such as Au and CdSe, despite the extensive investigations of their superlattices?Answering these questions requires a deeper understanding of multilayer diffraction. In what follows, we summarize our progress in rationalizing the origin of this phenomenon, at first through empirical observation and then by adapting the diffraction theory developed in the past for multilayer thin films, until we achieved a quantitative fitting of experimental diffraction patterns over extended angular ranges. By introducing the reader to the key advancements in our research, we provide answers to the questions above, we discuss what information can be extracted from patterns exhibiting collective interference effects, and we show how multilayer diffraction can provide insights into colloidal nanomaterials where other techniques struggle. Finally, with the help of literature patterns showing multilayer diffraction and simulations performed by us, we demonstrate that this collective diffraction effect is within reach for many appealing nanomaterials other than halide perovskites.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Toso S.; Baranov D.; Altamura D.; Scattarella F.; Dahl J.; Wang X.; Marras S.; Alivisatos A. P.; Singer A.; Giannini C.; Manna L. Multilayer Diffraction Reveals That Colloidal Superlattices Approach the Structural Perfection of Single Crystals. ACS Nano 2021, 15, 6243–6256. 10.1021/acsnano.0c08929. - DOI - PMC - PubMed
-
- Murray C. B.; Kagan C. R.; Bawendi M. G. Self-Organization of CdSe Nanocrystallites into Three-Dimensional Quantum Dot Superlattices. Science. 1995, 270, 1335–1338. 10.1126/science.270.5240.1335. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

