Cysteine-Selective Modification of Peptides and Proteins via Desulfurative C-C Bond Formation
- PMID: 36534955
- PMCID: PMC10946470
- DOI: 10.1002/chem.202202503
Cysteine-Selective Modification of Peptides and Proteins via Desulfurative C-C Bond Formation
Abstract
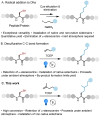
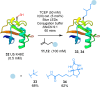
The site-selective modification of peptides and proteins facilitates the preparation of targeted therapeutic agents and tools to interrogate biochemical pathways. Among the numerous bioconjugation techniques developed to install groups of interest, those that generate C(sp3 )-C(sp3 ) bonds are significantly underrepresented despite affording proteolytically stable, biogenic linkages. Herein, a visible-light-mediated reaction is described that enables the site-selective modification of peptides and proteins via desulfurative C(sp3 )-C(sp3 ) bond formation. The reaction is rapid and high yielding in peptide systems, with comparable translation to proteins. Using this chemistry, a range of moieties is installed into model systems and an effective PTM-mimic is successfully integrated into a recombinantly expressed histone.
Keywords: bioconjugation; cysteine; desulfurization; post-translational modifications; site-selective.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

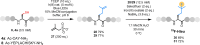

References
-
- Krall N., Da Cruz F. P., Boutureira O., Bernardes G. J. L., Nat. Chem. 2016, 8, 103–113. - PubMed
-
- Davis L., Chin J. W., Nat. Rev. Mol. 2012, 13, 168–182. - PubMed
-
- Wang L., Schultz P. G., Angew. Chem. Int. Ed. 2005, 44, 34–66; - PubMed
- Angew. Chem. 2005, 117, 34–68.
-
- Conibear A. C., Watson E. E., Payne R. J., Becker C. F., Chem. Soc. Rev. 2018, 47, 9046–9068. - PubMed
-
- Dawson P. E., Muir T. W., Clark-Lewis I., Kent S., Science 1994, 266, 776–779. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

