Pathophysiology and pharmacology of G protein-coupled receptors in the heart
- PMID: 36534965
- PMCID: PMC10202650
- DOI: 10.1093/cvr/cvac171
Pathophysiology and pharmacology of G protein-coupled receptors in the heart
Abstract
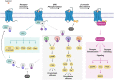
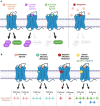
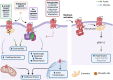
G protein-coupled receptors (GPCRs), comprising the largest superfamily of cell surface receptors, serve as fundamental modulators of cardiac health and disease owing to their key roles in the regulation of heart rate, contractile dynamics, and cardiac function. Accordingly, GPCRs are heavily pursued as drug targets for a wide variety of cardiovascular diseases ranging from heart failure, cardiomyopathy, and arrhythmia to hypertension and coronary artery disease. Recent advancements in understanding the signalling mechanisms, regulation, and pharmacological properties of GPCRs have provided valuable insights that will guide the development of novel therapeutics. Herein, we review the cellular signalling mechanisms, pathophysiological roles, and pharmacological developments of the major GPCRs in the heart, highlighting the β-adrenergic, muscarinic, and angiotensin receptors as exemplar subfamilies.
Keywords: Allosteric modulators; Biased signalling; G protein-coupled receptors; Heart failure.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: H.A.R. is a scientific cofounder of Trevena Inc., a company that is developing new GPCR ligands.
Figures



References
-
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 2002;3:639–650. - PubMed
-
- Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schioth HB. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 2006;88:263–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

