Dynamic phase separation of the androgen receptor and its coactivators key to regulate gene expression
- PMID: 36535377
- PMCID: PMC9841400
- DOI: 10.1093/nar/gkac1158
Dynamic phase separation of the androgen receptor and its coactivators key to regulate gene expression
Abstract
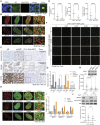
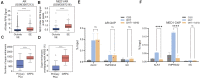
Numerous cancers, including prostate cancer (PCa), are addicted to transcription programs driven by specific genomic regions known as super-enhancers (SEs). The robust transcription of genes at such SEs is enabled by the formation of phase-separated condensates by transcription factors and coactivators with intrinsically disordered regions. The androgen receptor (AR), the main oncogenic driver in PCa, contains large disordered regions and is co-recruited with the transcriptional coactivator mediator complex subunit 1 (MED1) to SEs in androgen-dependent PCa cells, thereby promoting oncogenic transcriptional programs. In this work, we reveal that full-length AR forms foci with liquid-like properties in different PCa models. We demonstrate that foci formation correlates with AR transcriptional activity, as this activity can be modulated by changing cellular foci content chemically or by silencing MED1. AR ability to phase separate was also validated in vitro by using recombinant full-length AR protein. We also demonstrate that AR antagonists, which suppress transcriptional activity by targeting key regions for homotypic or heterotypic interactions of this receptor, hinder foci formation in PCa cells and phase separation in vitro. Our results suggest that enhanced compartmentalization of AR and coactivators may play an important role in the activation of oncogenic transcription programs in androgen-dependent PCa.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

