Efficacy of probiotic Streptococcus thermophilus in counteracting TGF-β1-induced fibrotic response in normal human dermal fibroblasts
- PMID: 36536411
- PMCID: PMC9764521
- DOI: 10.1186/s12950-022-00324-9
Efficacy of probiotic Streptococcus thermophilus in counteracting TGF-β1-induced fibrotic response in normal human dermal fibroblasts
Abstract
Background: Abnormal and deregulated skin wound healing associated with prolonged inflammation may result in dermal fibrosis. Since the current therapeutic strategies revealed unsatisfactory, the investigation of alternative approaches such as those based on the use of specific probiotic strains could provide promising therapeutic options. In this study, we aimed to evaluate whether the lysate from S. thermophilus could antagonize the fibrogenic effects of TGF-β1 in normal human dermal fibroblasts (NHDF).
Methods: NHDF were exposed to TGF-β1 to establish a fibrotic phenotype. Proliferation rate and cell number were measured using the IncuCyte® Live Cell Imager system and the trypan blue dye exclusion test. Phenoconversion markers (α-SMA and fibronectin) and collagen I levels were assessed by western blot and immunofluorescence. The mRNA levels of TGF-β1 were evaluated by RT-PCR. The Smad2/3 phosphorylation level as well as β-catenin and PPARγ expression, were assessed by western blot. The cell contractility function and migration of NHDF were studied using collagen gel retraction assay, and scratch wound healing assay, respectively. The effects of S. thermophilus lysate, alone or combined with TGF-β1, were evaluated on all of the above-listed parameters and markers associated with TGF-β1-induced fibrotic phenotype.
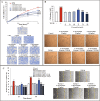
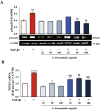
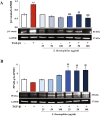
Results: Exposure to the S. thermophilus lysate significantly reduced the key mediators and events involved in the abnormal activation of myofibroblasts by TGF-β1 within the fibrotic profile. The S. thermophilus treatment significantly reduced cell proliferation, migration, and myo-differentiation. In addition, the treatment with probiotic lysate reduced the α-SMA, fibronectin, collagen-I expression levels, and affected the collagen contraction ability of activated dermal fibroblasts. Moreover, the probiotic targeted the TGF-β1 signaling, reducing Smad2/3 activation, TGF-β1 mRNA level, and β-catenin expression through the upregulation of PPARγ.
Conclusion: This is the first report showing that S. thermophilus lysate had a remarkable anti-fibrotic effect in TGF-β1-activated NHDF by inhibiting Smad signaling. Notably, the probiotic was able to reduce β-catenin and increase PPARγ levels. The findings support our point that S. thermophilus may help prevent or treat hypertrophic scarring and keloids.
Keywords: Fibrotic markers; PPARγ; S. thermophilus; Skin fibrosis; Smad signaling; TGF-β1; β-catenin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Soluble Fraction from Lysate of a High Concentration Multi-Strain Probiotic Formulation Inhibits TGF-β1-Induced Intestinal Fibrosis on CCD-18Co Cells.Nutrients. 2021 Mar 9;13(3):882. doi: 10.3390/nu13030882. Nutrients. 2021. PMID: 33803197 Free PMC article.
-
Streptococcus thermophilus CNCM I-5570 lysate counteracts the aging process in human dermal fibroblast cells by neutralizing harmful free radicals and impacting antioxidant and anti-inflammatory pathways, thus restoring their physiological functions.Biomed Pharmacother. 2025 Apr;185:117975. doi: 10.1016/j.biopha.2025.117975. Epub 2025 Mar 12. Biomed Pharmacother. 2025. PMID: 40081000
-
Human umbilical cord mesenchymal stem cell-derived exosomes suppress dermal fibroblasts-myofibroblats transition via inhibiting the TGF-β1/Smad 2/3 signaling pathway.Exp Mol Pathol. 2020 Aug;115:104468. doi: 10.1016/j.yexmp.2020.104468. Epub 2020 May 21. Exp Mol Pathol. 2020. PMID: 32445750
-
Wnt/β-catenin pathway forms a negative feedback loop during TGF-β1 induced human normal skin fibroblast-to-myofibroblast transition.J Dermatol Sci. 2012 Jan;65(1):38-49. doi: 10.1016/j.jdermsci.2011.09.012. Epub 2011 Oct 17. J Dermatol Sci. 2012. PMID: 22041457
-
Ursolic acid inhibits human dermal fibroblasts hyperproliferation, migration, and collagen deposition induced by TGF-β via regulating the Smad2/3 pathway.Gene. 2023 May 30;867:147367. doi: 10.1016/j.gene.2023.147367. Epub 2023 Mar 15. Gene. 2023. PMID: 36931410
Cited by
-
Probiotics in Wound Healing.Int J Mol Sci. 2024 May 24;25(11):5723. doi: 10.3390/ijms25115723. Int J Mol Sci. 2024. PMID: 38891909 Free PMC article. Review.
-
Decoding the impact of ageing and environment stressors on skin cell communication.Biogerontology. 2024 Oct 29;26(1):3. doi: 10.1007/s10522-024-10145-3. Biogerontology. 2024. PMID: 39470857 Review.
-
Skin Microbiota and Pathological Scars: A Bidirectional Two-Sample Mendelian Randomization Study.J Cosmet Dermatol. 2025 Feb;24(2):e16720. doi: 10.1111/jocd.16720. Epub 2024 Dec 9. J Cosmet Dermatol. 2025. PMID: 39654381 Free PMC article.
-
Evaluation of the Effectiveness of an Innovative Polycomponent Formulation on Adult and Aged Human Dermal Fibroblasts.Biomedicines. 2023 Aug 28;11(9):2410. doi: 10.3390/biomedicines11092410. Biomedicines. 2023. PMID: 37760851 Free PMC article.
-
Causal relationship between gut microbiota and pathological scars: a two-sample Mendelian randomization study.Front Med (Lausanne). 2024 Jul 2;11:1405097. doi: 10.3389/fmed.2024.1405097. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39015789 Free PMC article.
References
Grants and funding
- FFO MeSVA 2021 and 2022/Funding for research by Department of Life, Health & Environmental Sciences, University of L'Aquila
- FFO MeSVA 2021 and 2022/Funding for research by Department of Life, Health & Environmental Sciences, University of L'Aquila
- FFO MeSVA 2021 and 2022/Funding for research by Department of Life, Health & Environmental Sciences, University of L'Aquila
- FFO MeSVA 2021 and 2022/Funding for research by Department of Life, Health & Environmental Sciences, University of L'Aquila
- FFO MeSVA 2021 and 2022/Funding for research by Department of Life, Health & Environmental Sciences, University of L'Aquila
LinkOut - more resources
Full Text Sources

