3D printing of injury-preconditioned secretome/collagen/heparan sulfate scaffolds for neurological recovery after traumatic brain injury in rats
- PMID: 36536463
- PMCID: PMC9764714
- DOI: 10.1186/s13287-022-03208-0
3D printing of injury-preconditioned secretome/collagen/heparan sulfate scaffolds for neurological recovery after traumatic brain injury in rats
Abstract
Background: The effects of traumatic brain injury (TBI) can include physical disability and even death. The development of effective therapies to promote neurological recovery is still a challenging problem. 3D-printed biomaterials are considered to have a promising future in TBI repair. The injury-preconditioned secretome derived from human umbilical cord blood mesenchymal stem cells showed better stability in neurological recovery after TBI. Therefore, it is reasonable to assume that a biological scaffold loaded with an injury-preconditioned secretome could facilitate neural network reconstruction after TBI.
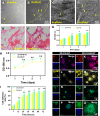
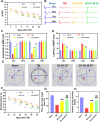
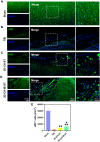
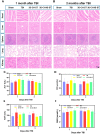
Methods: In this study, we fabricated injury-preconditioned secretome/collagen/heparan sulfate scaffolds by 3D printing. The scaffold structure and porosity were examined by scanning electron microscopy and HE staining. The cytocompatibility of the scaffolds was characterized by MTT analysis, HE staining and electron microscopy. The modified Neurological Severity Score (mNSS), Morris water maze (MWM), and motor evoked potential (MEP) were used to examine the recovery of cognitive and locomotor function after TBI in rats. HE staining, silver staining, Nissl staining, immunofluorescence, and transmission electron microscopy were used to detect the reconstruction of neural structures and pathophysiological processes. The biocompatibility of the scaffolds in vivo was characterized by tolerance exposure and liver/kidney function assays.
Results: The excellent mechanical and porosity characteristics of the composite scaffold allowed it to efficiently regulate the secretome release rate. MTT and cell adhesion assays demonstrated that the scaffold loaded with the injury-preconditioned secretome (3D-CH-IB-ST) had better cytocompatibility than that loaded with the normal secretome (3D-CH-ST). In the rat TBI model, cognitive and locomotor function including mNSS, MWM, and MEP clearly improved when the scaffold was transplanted into the damage site. There is a significant improvement in nerve tissue at the site of lesion. More abundant endogenous neurons with nerve fibers, synaptic structures, and myelin sheaths were observed in the 3D-CH-IB-ST group. Furthermore, the apoptotic response and neuroinflammation were significantly reduced and functional vessels were observed at the injury site. Good exposure tolerance in vivo demonstrated favorable biocompatibility of the scaffold.
Conclusions: Our results demonstrated that injury-preconditioned secretome/collagen/heparan sulfate scaffolds fabricated by 3D printing promoted neurological recovery after TBI by reconstructing neural networks, suggesting that the implantation of the scaffolds could be a novel way to alleviate brain damage following TBI.
Keywords: 3D printing; Biomaterial scaffolds; Human umbilical cord blood mesenchymal stem cells; Injury-preconditioned secretome; Neural reconstruction; Traumatic brain injury.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Shi W, Huang CJ, Xu XD, Jin GH, Huang RQ, Huang JF, et al. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater. 2016;45:247–261. doi: 10.1016/j.actbio.2016.09.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

