Zearalenone disturbs the reproductive-immune axis in pigs: the role of gut microbial metabolites
- PMID: 36536466
- PMCID: PMC9762105
- DOI: 10.1186/s40168-022-01397-7
Zearalenone disturbs the reproductive-immune axis in pigs: the role of gut microbial metabolites
Abstract
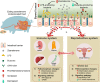
Background: Exposure to zearalenone (ZEN, a widespread Fusarium mycotoxin) causes reproductive toxicity and immunotoxicity in farm animals, and it then poses potential threats to human health through the food chain. A systematic understanding of underlying mechanisms on mycotoxin-induced toxicity is necessary for overcoming potential threats to farm animals and humans. The gastrointestinal tract is a first-line defense against harmful mycotoxins; however, it remains unknown whether mycotoxin (e.g., ZEN)-induced toxicity on the reproductive-immune axis is linked to altered gut microbial metabolites. In this study, using pigs (during the three phases) as an important large animal model, we investigated whether ZEN-induced toxicity on immune defense in the reproductive-immune axis was involved in altered gut microbial-derived metabolites. Moreover, we observed whether the regulation of gut microbial-derived metabolites through engineering ZEN-degrading enzymes counteracted ZEN-induced toxicity on the gut-reproductive-immune axis.
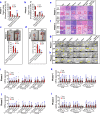
Results: Here, we showed ZEN exposure impaired immune defense in the reproductive-immune axis of pigs during phase 1/2. This impairment was accompanied by altered gut microbial-derived metabolites [e.g., decreased butyrate production, and increased lipopolysaccharides (LPS) production]. Reduction of butyrate production impaired the intestinal barrier via a GPR109A-dependent manner, and together with increased LPS in plasma then aggravated the systemic inflammation, thus directly and/or indirectly disturbing immune defense in the reproductive-immune axis. To validate these findings, we further generated recombinant Bacillus subtilis 168-expressing ZEN-degrading enzyme ZLHY-6 (the Bs-Z6 strain) as a tool to test the feasibility of enzymatic removal of ZEN from mycotoxin-contaminated food. Notably, modified gut microbial metabolites (e.g., butyrate, LPS) through the recombinant Bs-Z6 strain counteracted ZEN-induced toxicity on the intestinal barrier, thus enhancing immune defense in the reproductive-immune axis of pigs during phase-3. Also, butyrate supplementation restored ZEN-induced abnormalities in the porcine small intestinal epithelial cell.
Conclusions: Altogether, these results highlight the role of gut microbial-derived metabolites in ZEN-induced toxicity on the gut-reproductive-immune axis. Importantly, targeting these gut microbial-derived metabolites opens a new window for novel preventative strategies or therapeutic interventions for mycotoxicosis associated to ZEN.
Keywords: Butyrate; Gastrointestinal tract; Immune system; Immunity; Lipopolysaccharides; Mycoestrogen; Mycotoxin; Reproductive system; Sus scrofa; Zearalenone.
© 2022. The Author(s).
Conflict of interest statement
Lin Zhou was employed by Shenzhen Premix INVE Nutrition, Co., LTD. The remaining authors declare that they have no competing interests.
Figures






Similar articles
-
Zearalenone-induced hepatointestinal toxicity in laying hens: unveiling the role of gut microbiota and fecal metabolites.Poult Sci. 2024 Nov;103(11):104221. doi: 10.1016/j.psj.2024.104221. Epub 2024 Aug 16. Poult Sci. 2024. PMID: 39241615 Free PMC article.
-
Short-term consumption of the mycotoxin zearalenone by pubertal gilts causes persistent changes in the histoarchitecture of reproductive tissues.J Anim Sci. 2023 Jan 3;101:skac421. doi: 10.1093/jas/skac421. J Anim Sci. 2023. PMID: 36574505 Free PMC article.
-
Enzymatic Degradation of Zearalenone in the Gastrointestinal Tract of Pigs, Chickens, and Rainbow Trout.Toxins (Basel). 2023 Jan 6;15(1):48. doi: 10.3390/toxins15010048. Toxins (Basel). 2023. PMID: 36668868 Free PMC article.
-
Mycotoxin: Its Impact on Gut Health and Microbiota.Front Cell Infect Microbiol. 2018 Feb 26;8:60. doi: 10.3389/fcimb.2018.00060. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29535978 Free PMC article. Review.
-
Structure-toxicity relationships, toxicity mechanisms and health risk assessment of food-borne modified deoxynivalenol and zearalenone: A comprehensive review.Sci Total Environ. 2022 Feb 1;806(Pt 3):151192. doi: 10.1016/j.scitotenv.2021.151192. Epub 2021 Oct 25. Sci Total Environ. 2022. PMID: 34710421 Review.
Cited by
-
β-Nicotinamide Mononucleotide Alleviates Hydrogen Peroxide-Induced Cell Cycle Arrest and Death in Ovarian Granulosa Cells.Int J Mol Sci. 2023 Oct 27;24(21):15666. doi: 10.3390/ijms242115666. Int J Mol Sci. 2023. PMID: 37958650 Free PMC article.
-
A Novel Bacillus Velezensis for Efficient Degradation of Zearalenone.Foods. 2024 Feb 9;13(4):530. doi: 10.3390/foods13040530. Foods. 2024. PMID: 38397507 Free PMC article.
-
The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective.Front Immunol. 2023 Oct 9;14:1277102. doi: 10.3389/fimmu.2023.1277102. eCollection 2023. Front Immunol. 2023. PMID: 37876938 Free PMC article. Review.
-
Zearalenone Exposure Disrupts STAT-ISG15 in Rat Colon: A Potential Linkage between Zearalenone and Inflammatory Bowel Disease.Toxins (Basel). 2023 Jun 9;15(6):392. doi: 10.3390/toxins15060392. Toxins (Basel). 2023. PMID: 37368693 Free PMC article.
-
Effects of Exposure to Low Zearalenone Concentrations Close to the EU Recommended Value on Weaned Piglets' Colon.Toxins (Basel). 2023 Mar 9;15(3):206. doi: 10.3390/toxins15030206. Toxins (Basel). 2023. PMID: 36977097 Free PMC article.
References
-
- Gratz SW, Dinesh R, Yoshinari T, Holtrop G, Richardson AJ, Duncan G, et al. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro. Mol Nutr Food Res. 2017;61(4). 10.1002/mnfr.201600680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

