Dynamics and Equilibration Mechanisms in Block Copolymer Particles
- PMID: 36536887
- PMCID: PMC9756915
- DOI: 10.1021/acspolymersau.2c00033
Dynamics and Equilibration Mechanisms in Block Copolymer Particles
Abstract
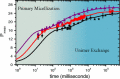
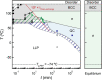
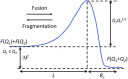
Self-assembly of block copolymers into interesting and useful nanostructures, in both solution and bulk, is a vibrant research arena. While much attention has been paid to characterization and prediction of equilibrium phases, the associated dynamic processes are far from fully understood. Here, we explore what is known and not known about the equilibration of particle phases in the bulk, and spherical micelles in solution. The presumed primary equilibration mechanisms are chain exchange, fusion, and fragmentation. These processes have been extensively studied in surfactants and lipids, where they occur on subsecond time scales. In contrast, increased chain lengths in block copolymers create much larger barriers, and time scales can become prohibitively slow. In practice, equilibration of block copolymers is achievable only in proximity to the critical micelle temperature (in solution) or the order-disorder transition (in the bulk). Detailed theories for these processes in block copolymers are few. In the bulk, the rate of chain exchange can be quantified by tracer diffusion measurements. Often the rate of equilibration, in terms of number density and aggregation number of particles, is much slower than chain exchange, and consequently observed particle phases are often metastable. This is particularly true in regions of the phase diagram where Frank-Kasper phases occur. Chain exchange in solution has been explored quantitatively by time-resolved SANS, but the results are not well captured by theory. Computer simulations, particularly via dissipative particle dynamics, are beginning to shed light on the chain escape mechanism at the molecular level. The rate of fragmentation has been quantified in a few experimental systems, and TEM images support a mechanism akin to the anaphase stage of mitosis in cells, via a thin neck that pinches off to produce two smaller micelles. Direct measurements of micelle fusion are quite rare. Suggestions for future theoretical, computational, and experimental efforts are offered.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
















References
-
- Gohy J.-F. Block Copolymer Micelles. Adv. Polym. Sci. 2005, 190, 65–136. 10.1007/12_048. - DOI
-
- Tritschler U.; Pearce S.; Gwyther J.; Whittell G. R.; Manners I. 50th Anniversary Perspective: Functional Nanoparticles from the Solution Self-Assembly of Block Copolymers. Macromolecules 2017, 50 (9), 3439–3463. 10.1021/acs.macromol.6b02767. - DOI
-
- Bates C. M.; Bates F. S. 50th Anniversary Perspective: Block Polymers—Pure Potential. Macromolecules 2017, 50 (1), 3–22. 10.1021/acs.macromol.6b02355. - DOI
Publication types
LinkOut - more resources
Full Text Sources
