Galectin-3 interacts with PD-1 and counteracts the PD-1 pathway-driven regulation of T cell and osteoclast activity in Rheumatoid Arthritis
- PMID: 36537046
- PMCID: PMC10078345
- DOI: 10.1111/sji.13245
Galectin-3 interacts with PD-1 and counteracts the PD-1 pathway-driven regulation of T cell and osteoclast activity in Rheumatoid Arthritis
Abstract
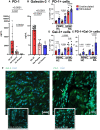
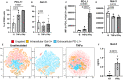
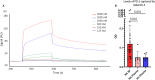
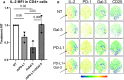
Rheumatoid arthritis (RA) is an autoimmune disease characterized by joint inflammation and bone erosions. The glycosylated programmed death-1 (PD-1) receptor plays an important role in regulating immune responses and maintaining tolerance. In this study, we focus on two features observed in RA: impaired PD-1 signalling and Galectin-3 (Gal-3) upregulation. We hypothesize that Gal-3 binds PD-1 and PD-1 ligands, potentially contributing to impaired PD-1 signalling. PD-1 and Gal-3 levels in RA synovial fluid (SF) and plasma were evaluated by ELISA. PD-1 and Gal-3 interaction was examined by Surface Plasmon Resonance and ELISA. PD-1, PD-L1 and Gal-3 expression on mononuclear cells from SF and peripheral blood as well as fibroblast-like synoviocytes were examined by flow cytometry. Effects of Gal-3 and PD-L1 on osteoclast formation was evaluated by tartrate-resistant acid phosphatase assay. We show that Gal-3 binds PD-1 and PD-L1. Results demonstrated high expression of PD-1 and Gal-3 on mononuclear cells, especially from SF. Gal-3 inhibited PD-1 signalling when PD-L1 was present. Furthermore, a role of Gal-3 in osteoclast formation was observed in vitro, both directly but also through PD-1:PD-L1 inhibition. Effects of Gal-3 on the PD-1 signalling axis are proposed to be inhibitory, meaning high Gal-3 levels in the complex synovial microenvironment are not desirable in RA. Preventing Gal-3's inhibitory role on PD-1 signalling could, therefore, be a therapeutic target in RA by affecting inflammatory T cell responses and osteoclasts.
Keywords: Galectin-3; PD-1; autoimmunity; co-inhibitory receptors; inflammation; rheumatoid arthritis.
© 2022 The Authors. Scandinavian Journal of Immunology published by John Wiley & Sons Ltd on behalf of The Scandinavian Foundation for Immunology.
Conflict of interest statement
Authors have no disclosures or competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

