Body mass index and childhood symptoms of depression, anxiety, and attention-deficit hyperactivity disorder: A within-family Mendelian randomization study
- PMID: 36537070
- PMCID: PMC9767454
- DOI: 10.7554/eLife.74320
Body mass index and childhood symptoms of depression, anxiety, and attention-deficit hyperactivity disorder: A within-family Mendelian randomization study
Abstract
Background: Higher BMI in childhood is associated with emotional and behavioural problems, but these associations may not be causal. Results of previous genetic studies imply causal effects but may reflect influence of demography and the family environment.
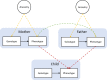
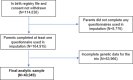
Methods: This study used data on 40,949 8-year-old children and their parents from the Norwegian Mother, Father and Child Cohort Study (MoBa) and Medical Birth Registry of Norway (MBRN). We investigated the impact of BMI on symptoms of depression, anxiety, and attention-deficit hyperactivity disorder (ADHD) at age 8. We applied within-family Mendelian randomization, which accounts for familial effects by controlling for parental genotype.
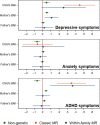
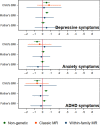
Results: Within-family Mendelian randomization estimates using genetic variants associated with BMI in adults suggested that a child's own BMI increased their depressive symptoms (per 5 kg/m2 increase in BMI, beta = 0.26 S.D., CI = -0.01,0.52, p=0.06) and ADHD symptoms (beta = 0.38 S.D., CI = 0.09,0.63, p=0.009). These estimates also suggested maternal BMI, or related factors, may independently affect a child's depressive symptoms (per 5 kg/m2 increase in maternal BMI, beta = 0.11 S.D., CI:0.02,0.09, p=0.01). However, within-family Mendelian randomization using genetic variants associated with retrospectively-reported childhood body size did not support an impact of BMI on these outcomes. There was little evidence from any estimate that the parents' BMI affected the child's ADHD symptoms, or that the child's or parents' BMI affected the child's anxiety symptoms.
Conclusions: We found inconsistent evidence that a child's BMI affected their depressive and ADHD symptoms, and little evidence that a child's BMI affected their anxiety symptoms. There was limited evidence of an influence of parents' BMI. Genetic studies in samples of unrelated individuals, or using genetic variants associated with adult BMI, may have overestimated the causal effects of a child's own BMI.
Funding: This research was funded by the Health Foundation. It is part of the HARVEST collaboration, supported by the Research Council of Norway. Individual co-author funding: the European Research Council, the South-Eastern Norway Regional Health Authority, the Research Council of Norway, Helse Vest, the Novo Nordisk Foundation, the University of Bergen, the South-Eastern Norway Regional Health Authority, the Trond Mohn Foundation, the Western Norway Regional Health Authority, the Norwegian Diabetes Association, the UK Medical Research Council. The Medical Research Council (MRC) and the University of Bristol support the MRC Integrative Epidemiology Unit.
Keywords: MBRN; Mendelian randomization; MoBa; Within-family; body mass index; epidemiology; genetics; genomics; global health; human; within-families.
Plain language summary
Some studies show that children with obesity are more likely to receive a diagnosis of depression, anxiety, or attention-deficit hyperactivity disorder (ADHD). But this does not necessarily mean obesity causes these conditions. Depression, anxiety, or ADHD could cause obesity. A child's environment, including family income or their parents' mental health, could also affect a child's weight and mental health. Understanding the nature of these relationships could help scientists develop better interventions for both obesity and mental health conditions. Genetic studies may help scientists better understand the role of the environment in these conditions, but it's important to consider both the child's and their parents’ genetics in these analyses. This is because parents and children share not only genes, but also environmental conditions. For example, families that carry genetic variants associated with higher body weight might also have lower incomes, if parents have been affected by biases against heavier people in society and the workplace. Children in these families could have worse mental health because of effects of their parent’s weight, rather than their own weight. Looking at both child and adult genetics can help disentangle these processes. Hughes et al. show that a child's own body mass index, a ratio of weight and height, is not strongly associated with the child’s mental health symptoms. They analysed genetic, weight, and health survey data from about 41,000 8-year-old children and their parents. The results suggest that a child's own BMI does not have a large effect on their anxiety symptoms. There was also no clear evidence that a child's BMI affected their symptoms of depression or ADHD. These results contradict previous studies, which did not account for parental genetics. Hughes et al. suggest that, at least for eight-year-olds, factors linked with adult weight and which differ between families may be more critical to a child's mental health than a child’s own weight. For older children and adolescents, this may not be the case, and the individual’s own weight may be more important. As a result, policies designed to reduce obesity in mid-childhood are unlikely to greatly improve the mental health of children. On the other hand, policies targeting the environmental or societal factors contributing to higher body weights, bias against people with higher weights, and poor child mental health directly may be more beneficial.
© 2022, Hughes et al.
Conflict of interest statement
AH, ES, TM, ZA, MT, HA, TR, PM, ØH, SJ, PN, GD, AH, LH, ND No competing interests declared, OA has received speaker’s honorarium from Sunovion and Lundbeck and is a consultant for HealthLytix
Figures


Similar articles
-
The effectiveness of web-based programs on the reduction of childhood obesity in school-aged children: A systematic review.JBI Libr Syst Rev. 2012;10(42 Suppl):1-14. doi: 10.11124/jbisrir-2012-248. JBI Libr Syst Rev. 2012. PMID: 27820152
-
Health-related quality of life in children and adolescents who have a diagnosis of attention-deficit/hyperactivity disorder.Pediatrics. 2004 Nov;114(5):e541-7. doi: 10.1542/peds.2004-0844. Pediatrics. 2004. PMID: 15520087
-
Mechanisms linking parental educational attainment with child ADHD, depression, and academic problems: a study of extended families in The Norwegian Mother, Father and Child Cohort Study.J Child Psychol Psychiatry. 2020 Sep;61(9):1009-1018. doi: 10.1111/jcpp.13197. Epub 2020 Jan 19. J Child Psychol Psychiatry. 2020. PMID: 31957030 Free PMC article.
-
Do parents and children agree on rating a child's HRQOL? A systematic review and Meta-analysis of comparisons between children with attention deficit hyperactivity disorder and children with typical development using the PedsQLTM.Disabil Rehabil. 2019 Feb;41(3):265-275. doi: 10.1080/09638288.2017.1391338. Epub 2017 Oct 23. Disabil Rehabil. 2019. PMID: 29057670
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Mood instability and low back pain: a mendelian randomization study.Front Neurol. 2023 Sep 15;14:1252329. doi: 10.3389/fneur.2023.1252329. eCollection 2023. Front Neurol. 2023. PMID: 37786864 Free PMC article.
-
The Impact of Parental Behaviors on Children's Lifestyle, Dietary Habits, Screen Time, Sleep Patterns, Mental Health, and BMI: A Scoping Review.Children (Basel). 2025 Feb 8;12(2):203. doi: 10.3390/children12020203. Children (Basel). 2025. PMID: 40003305 Free PMC article. Review.
-
Mendelian randomization studies of depression: evidence, opportunities, and challenges.Ann Gen Psychiatry. 2023 Nov 23;22(1):47. doi: 10.1186/s12991-023-00479-6. Ann Gen Psychiatry. 2023. PMID: 37996851 Free PMC article. Review.
-
Investigating the causal effects of childhood and adulthood adiposity on later life mental health outcome: a Mendelian randomization study.BMC Med. 2025 Jan 6;23(1):4. doi: 10.1186/s12916-024-03765-6. BMC Med. 2025. PMID: 39757155 Free PMC article.
-
A Causal Inference Study of Circulating Metabolites Mediating the Effect of Obesity-Related Indicators on the Incidence of Anxiety Disorders.Brain Behav. 2025 Jul;15(7):e70653. doi: 10.1002/brb3.70653. Brain Behav. 2025. PMID: 40619960 Free PMC article.
References
-
- Bahrami S, Steen NE, Shadrin A, O’Connell K, Frei O, Bettella F, Wirgenes KV, Krull F, Fan CC, Dale AM, Smeland OB, Djurovic S, Andreassen OA. Shared genetic loci between body mass index and major psychiatric disorders: a genome-wide association study. JAMA Psychiatry. 2020;77:503–512. doi: 10.1001/jamapsychiatry.2019.4188. - DOI - PMC - PubMed
-
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the screen for child anxiety related emotional disorders (scared): a replication study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1230–1236. doi: 10.1097/00004583-199910000-00011. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

