Multiparameter flow cytometry in the evaluation of myelodysplasia: Analytical issues: Recommendations from the European LeukemiaNet/International Myelodysplastic Syndrome Flow Cytometry Working Group
- PMID: 36537621
- PMCID: PMC10107708
- DOI: 10.1002/cyto.b.22108
Multiparameter flow cytometry in the evaluation of myelodysplasia: Analytical issues: Recommendations from the European LeukemiaNet/International Myelodysplastic Syndrome Flow Cytometry Working Group
Abstract
Multiparameter flow cytometry (MFC) is one of the essential ancillary methods in bone marrow (BM) investigation of patients with cytopenia and suspected myelodysplastic syndrome (MDS). MFC can also be applied in the follow-up of MDS patients undergoing treatment. This document summarizes recommendations from the International/European Leukemia Net Working Group for Flow Cytometry in Myelodysplastic Syndromes (ELN iMDS Flow) on the analytical issues in MFC for the diagnostic work-up of MDS. Recommendations for the analysis of several BM cell subsets such as myeloid precursors, maturing granulocytic and monocytic components and erythropoiesis are given. A core set of 17 markers identified as independently related to a cytomorphologic diagnosis of myelodysplasia is suggested as mandatory for MFC evaluation of BM in a patient with cytopenia. A myeloid precursor cell (CD34+ CD19- ) count >3% should be considered immunophenotypically indicative of myelodysplasia. However, MFC results should always be evaluated as part of an integrated hematopathology work-up. Looking forward, several machine-learning-based analytical tools of interest should be applied in parallel to conventional analytical methods to investigate their usefulness in integrated diagnostics, risk stratification, and potentially even in the evaluation of response to therapy, based on MFC data. In addition, compiling large uniform datasets is desirable, as most of the machine-learning-based methods tend to perform better with larger numbers of investigated samples, especially in such a heterogeneous disease as MDS.
Keywords: ELN; consensus; flow cytometry; myelodysplastic syndromes; standardization.
© 2022 The Authors. Cytometry Part B: Clinical Cytometry published by Wiley Periodicals LLC on behalf of International Clinical Cytometry Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
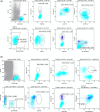
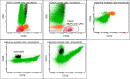
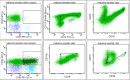

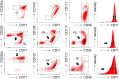
References
-
- Aalbers, A. M. , van den Heuvel‐Eibrink, M. M. , Baumann, I. , Dworzak, M. , Hasle, H. , Locatelli, F. , De Moerloose, B. , Schmugge, M. , Mejstrikova, E. , Nováková, M. , Zecca, M. , Zwaan, C. M. , Te Marvelde, J. G. , Langerak, A. W. , van Dongen, J. J. , Pieters, R. , Niemeyer, C. M. , & van der Velden, V. H. (2015). Bone marrow immunophenotyping by flow cytometry in refractory cytopenia of childhood. Haematologica, 100(3), 315–323. 10.3324/haematol.2014.107706 - DOI - PMC - PubMed
-
- Aalbers, A. M. , van den Heuvel‐Eibrink, M. M. , de Haas, V. , Te Marvelde, J. G. , de Jong, A. X. , van der Burg, M. , Dworzak, M. , Hasle, H. , Locatelli, F. , De Moerloose, B. , Schmugge, M. , Stary, J. , Zecca, M. , Zwaan, C. M. , van de Loosdrecht, A. A. , van Dongen, J. J. , Niemeyer, C. M. , & van der Velden, V. H. (2013). Applicability of a reproducible flow cytometry scoring system in the diagnosis of refractory cytopenia of childhood. Leukemia, 27(9), 1923–1925. 10.1038/leu.2013.81 - DOI - PubMed
-
- Alhan, C. , Westers, T. M. , Cremers, E. M. , Cali, C. , Witte, B. I. , Ossenkoppele, G. J. , & van de Loosdrecht, A. A. (2014). High flow cytometric scores identify adverse prognostic subgroups within the revised international prognostic scoring system for myelodysplastic syndromes. British Journal of Haematology, 167(1), 100–109. 10.1111/bjh.12994 - DOI - PubMed
-
- Alhan, C. , Westers, T. M. , Cremers, E. M. , Cali, C. , Witte, B. I. , Ossenkoppele, G. J. , & van de Loosdrecht, A. A. (2016). The myelodysplastic syndromes flow cytometric score: A three‐parameter prognostic flow cytometric scoring system. Leukemia, 30(3), 658–665. 10.1038/leu.2015.295 - DOI - PubMed
-
- Alhan, C. , Westers, T. M. , van der Helm, L. H. , Eeltink, C. , Huls, G. , Witte, B. I. , Buchi, F. , Santini, V. , Ossenkoppele, G. J. , & van de Loosdrecht, A. A. (2014). Absence of aberrant myeloid progenitors by flow cytometry is associated with favorable response to azacitidine in higher risk myelodysplastic syndromes. Cytometry. Part B: Clinical Cytometry, 86(3), 207–215. 10.1002/cyto.b.21160 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

