Consumption of 2 Green Kiwifruits Daily Improves Constipation and Abdominal Comfort-Results of an International Multicenter Randomized Controlled Trial
- PMID: 36537785
- PMCID: PMC10226473
- DOI: 10.14309/ajg.0000000000002124
Consumption of 2 Green Kiwifruits Daily Improves Constipation and Abdominal Comfort-Results of an International Multicenter Randomized Controlled Trial
Abstract
Introduction: Consumption of green kiwifruit is known to relieve constipation. Previous studies have also reported improvements in gastrointestinal (GI) comfort. We investigated the effect of consuming green kiwifruit on GI function and comfort.
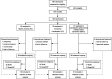
Methods: Participants included healthy controls (n = 63), patients with functional constipation (FC, n = 60), and patients with constipation-predominant irritable bowel syndrome (IBS-C, n = 61) randomly assigned to consume 2 green kiwifruits or psyllium (7.5 g) per day for 4 weeks, followed by a 4-week washout, and then the other treatment for 4 weeks. The primary outcome was the number of complete spontaneous bowel movements (CSBM) per week. Secondary outcomes included GI comfort which was measured using the GI symptom rating scale, a validated instrument. Data (intent-to-treat) were analyzed as difference from baseline using repeated measures analysis of variance suitable for AB/BA crossover design.
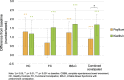
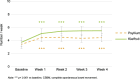
Results: Consumption of green kiwifruit was associated with a clinically relevant increase of ≥ 1.5 CSBM per week (FC; 1.53, P < 0.0001, IBS-C; 1.73, P = 0.0003) and significantly improved measures of GI comfort (GI symptom rating scale total score) in constipated participants (FC, P < 0.0001; IBS-C, P < 0.0001). No significant adverse events were observed.
Discussion: This study provides original evidence that the consumption of a fresh whole fruit has demonstrated clinically relevant increases in CSBM and improved measures of GI comfort in constipated populations. Green kiwifruits are a suitable dietary treatment for relief of constipation and associated GI comfort.
Trial registration: ClinicalTrials.gov NCT02888392.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures



Comment in
-
In persons with constipation or IBS-C, kiwifruit vs. psyllium increased spontaneous bowel movements.Ann Intern Med. 2023 May;176(5):JC53. doi: 10.7326/J23-0022. Epub 2023 May 2. Ann Intern Med. 2023. PMID: 37126812
References
-
- Aziz I, Palsson OS, Törnblom H, et al. . The prevalence and impact of overlapping Rome IV-diagnosed functional gastrointestinal disorders on somatization, quality of life, and healthcare utilization: A cross-sectional general population study in three countries. Am J Gastroenterol 2018;113(1):86–96. - PubMed
-
- Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology 2021;160(1):99–114.e3. - PubMed
-
- Drossman DA. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016;150(6):1262–79.e2. - PubMed
-
- Drossman DA, Hasler WL. Rome IV-functional GI disorders: Disorders of gut-brain interaction. Gastroenterology 2016;150(6):1257–61. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

