Impaired muscle stem cell function and abnormal myogenesis in acquired myopathies
- PMID: 36538023
- PMCID: PMC9829652
- DOI: 10.1042/BSR20220284
Impaired muscle stem cell function and abnormal myogenesis in acquired myopathies
Abstract
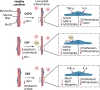
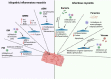
Skeletal muscle possesses a high plasticity and a remarkable regenerative capacity that relies mainly on muscle stem cells (MuSCs). Molecular and cellular components of the MuSC niche, such as immune cells, play key roles to coordinate MuSC function and to orchestrate muscle regeneration. An abnormal infiltration of immune cells and/or imbalance of pro- and anti-inflammatory cytokines could lead to MuSC dysfunctions that could have long lasting effects on muscle function. Different genetic variants were shown to cause muscular dystrophies that intrinsically compromise MuSC function and/or disturb their microenvironment leading to impaired muscle regeneration that contributes to disease progression. Alternatively, many acquired myopathies caused by comorbidities (e.g., cardiopulmonary or kidney diseases), chronic inflammation/infection, or side effects of different drugs can also perturb MuSC function and their microenvironment. The goal of this review is to comprehensively summarize the current knowledge on acquired myopathies and their impact on MuSC function. We further describe potential therapeutic strategies to restore MuSC regenerative capacity.
Keywords: inflammation; muscle stem cell; myogenesis; myopathies; regeneration; therapeutics.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

