Are there foetal extracellular vesicles in maternal blood? Prospects for diagnostic biomarker discovery
- PMID: 36538060
- PMCID: PMC9977902
- DOI: 10.1007/s00109-022-02278-0
Are there foetal extracellular vesicles in maternal blood? Prospects for diagnostic biomarker discovery
Abstract
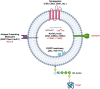
Prenatal diagnosis of congenital disease improves clinical outcomes; however, as many as 50% of congenital heart disease cases are missed by current ultrasound screening methods. This indicates a need for improved screening technology. Extracellular vesicles (EVs) have attracted enormous interest in recent years for their potential in diagnostics. EVs mediate endocrine signalling in health and disease and are known to regulate aspects of embryonic development. Here, we critically evaluate recent evidence suggesting that EVs released from the foetus are able to cross the placenta and enter the maternal circulation. Furthermore, EVs from the mother appear to be transported in the reverse direction, whilst the placenta itself acts as a source of EVs. Experimental work utilising rodent models employing either transgenically encoded reporters or application of fluorescent tracking dyes provide convincing evidence of foetal-maternal crosstalk. This is supported by clinical data demonstrating expression of placental-origin EVs in maternal blood, as well as limited evidence for the presence of foetal-origin EVs. Together, this work raises the possibility that foetal EVs present in maternal blood could be used for the diagnosis of congenital disease. We discuss the challenges faced by researchers in translating these basic science findings into a clinical non-invasive prenatal test.
Keywords: Congenital disease; Diagnostics; Exosome; Extracellular vesicle; Microvesicle; Placenta; Trophoblast.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Varghese SE, El Otol RHM, Al Olama FS, Elbadawi SAM. The importance of early detection of genetic diseases. Dubai Medical Journal. 2021;4:133–141. doi: 10.1159/000514215. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

