Serological response to COVID-19 pneumonia and increasing severity over 18 months in a prospective cohort of hospitalized patients
- PMID: 36538188
- PMCID: PMC9765378
- DOI: 10.1007/s11739-022-03177-5
Serological response to COVID-19 pneumonia and increasing severity over 18 months in a prospective cohort of hospitalized patients
Abstract
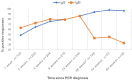
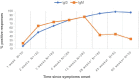
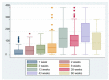
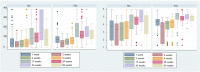
In this study, we present an 18-month serological follow-up of 294 patients with COVID-19 pneumonia. The aim was to assess the dynamics of serological response and its correlation with clinical worsening, as well as to describe clinical worsening determinants. Results of the study showed an early immunoglobulin M response, which clearly diminished starting at 4 months, but nonetheless, a small group of patients remained positive. As for immunoglobulin G, levels were higher up to 6 months in patients who presented clinical worsening during hospitalization. High titers of the immunoglobulin were maintained in all patients during follow-up, which would indicate that humoral immunity due to infection is long-lasting. Male sex, presence of myalgias and extensive radiological affectation were significantly correlated with clinical worsening.
Keywords: COVID-19 pneumonia; Clinical worsening; SARS-COV-2; Serological response.
© 2022. The Author(s), under exclusive licence to Società Italiana di Medicina Interna (SIMI).
Conflict of interest statement
The authors declare that there are no competing interests.
Figures

References
-
- Burrell CJ, Howard CR, Murphy FA. Laboratory diagnosis of virus diseases. Fenner and White’s Med Virol. 2017;1:135–154. doi: 10.1016/B978-0-12-375156-0.00010-2. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

