State-of-the-art analytical methods of viral infections in human lung organoids
- PMID: 36538516
- PMCID: PMC9767351
- DOI: 10.1371/journal.pone.0276115
State-of-the-art analytical methods of viral infections in human lung organoids
Erratum in
-
Correction: State-of-the-art analytical methods of viral infections in human lung organoids.PLoS One. 2023 Nov 3;18(11):e0294216. doi: 10.1371/journal.pone.0294216. eCollection 2023. PLoS One. 2023. PMID: 37922305 Free PMC article.
Abstract
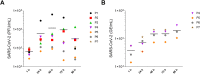
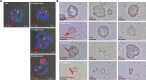
Human-based organ models can provide strong predictive value to investigate the tropism, virulence, and replication kinetics of viral pathogens. Currently, such models have received widespread attention in the study of SARS-CoV-2 causing the COVID-19 pandemic. Applicable to a large set of organoid models and viruses, we provide a step-by-step work instruction for the infection of human alveolar-like organoids with SARS-CoV-2 in this protocol collection. We also prepared a detailed description on state-of-the-art methodologies to assess the infection impact and the analysis of relevant host factors in organoids. This protocol collection consists of five different sets of protocols. Set 1 describes the protein extraction from human alveolar-like organoids and the determination of protein expression of angiotensin-converting enzyme 2 (ACE2), transmembrane serine protease 2 (TMPRSS2) and FURIN as exemplary host factors of SARS-CoV-2. Set 2 provides detailed guidance on the extraction of RNA from human alveolar-like organoids and the subsequent qPCR to quantify the expression level of ACE2, TMPRSS2, and FURIN as host factors of SARS-CoV-2 on the mRNA level. Protocol set 3 contains an in-depth explanation on how to infect human alveolar-like organoids with SARS-CoV-2 and how to quantify the viral replication by plaque assay and viral E gene-based RT-qPCR. Set 4 provides a step-by-step protocol for the isolation of single cells from infected human alveolar-like organoids for further processing in single-cell RNA sequencing or flow cytometry. Set 5 presents a detailed protocol on how to perform the fixation of human alveolar-like organoids and guides through all steps of immunohistochemistry and in situ hybridization to visualize SARS-CoV-2 and its host factors. The infection and all subsequent analytical methods have been successfully validated by biological replications with human alveolar-like organoids based on material from different donors.
Copyright: © 2022 Baumgardt et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

