Acute high-fat diet impairs macrophage-supported intestinal damage resolution
- PMID: 36538527
- PMCID: PMC9977439
- DOI: 10.1172/jci.insight.164489
Acute high-fat diet impairs macrophage-supported intestinal damage resolution
Abstract
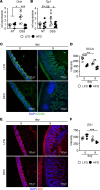
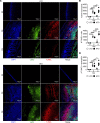
Chronic exposure to high-fat diets (HFD) worsens intestinal disease pathology, but acute effects of HFD in tissue damage remain unclear. Here, we used short-term HFD feeding in a model of intestinal injury and found sustained damage with increased cecal dead neutrophil accumulation, along with dietary lipid accumulation. Neutrophil depletion rescued enhanced pathology. Macrophages from HFD-treated mice showed reduced capacity to engulf dead neutrophils. Macrophage clearance of dead neutrophils activates critical barrier repair and antiinflammatory pathways, including IL-10, which was lost after acute HFD feeding and intestinal injury. IL-10 overexpression restored intestinal repair after HFD feeding and intestinal injury. Macrophage exposure to lipids from the HFD prevented tethering and uptake of apoptotic cells and Il10 induction. Milk fat globule-EGF factor 8 (MFGE8) is a bridging molecule that facilitates macrophage uptake of dead cells. MFGE8 also facilitates lipid uptake, and we demonstrate that dietary lipids interfere with MFGE8-mediated macrophage apoptotic neutrophil uptake and subsequent Il10 production. Our findings demonstrate that HFD promotes intestinal pathology by interfering with macrophage clearance of dead neutrophils, leading to unresolved tissue damage.
Keywords: Immunology; Macrophages; Neutrophils.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

