Local neuroplasticity in adult glaucomatous visual cortex
- PMID: 36539453
- PMCID: PMC9767937
- DOI: 10.1038/s41598-022-24709-1
Local neuroplasticity in adult glaucomatous visual cortex
Abstract
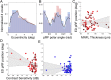
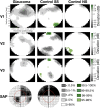
The degree to which the adult human visual cortex retains the ability to functionally adapt to damage at the level of the eye remains ill-understood. Previous studies on cortical neuroplasticity primarily focused on the consequences of foveal visual field defects (VFD), yet these findings may not generalize to peripheral defects such as occur in glaucoma. Moreover, recent findings on neuroplasticity are often based on population receptive field (pRF) mapping, but interpreting these results is complicated in the absence of appropriate control conditions. Here, we used fMRI-based neural modeling to assess putative changes in pRFs associated with glaucomatous VFD. We compared the fMRI-signals and pRF in glaucoma participants to those of controls with case-matched simulated VFD. We found that the amplitude of the fMRI-signal is reduced in glaucoma compared to control participants and correlated with disease severity. Furthermore, while coarse retinotopic structure is maintained in all participants with glaucoma, we observed local pRF shifts and enlargements in early visual areas, relative to control participants. These differences suggest that the adult brain retains some degree of local neuroplasticity. This finding has translational relevance, as it is consistent with VFD masking, which prevents glaucoma patients from noticing their VFD and seeking timely treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

