Development and Validation of a Western Blot Method to Quantify Mini-Dystrophin in Human Skeletal Muscle Biopsies
- PMID: 36539515
- PMCID: PMC10034579
- DOI: 10.1208/s12248-022-00776-0
Development and Validation of a Western Blot Method to Quantify Mini-Dystrophin in Human Skeletal Muscle Biopsies
Abstract
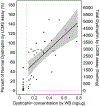
Duchenne muscular dystrophy (DMD) is a degenerative muscular disease affecting roughly one in 5000 males at birth. The disease is often caused by inherited X-linked recessive pathogenic variants in the dystrophin gene, but may also arise from de novo mutations. Disease-causing variants include nonsense, out of frame deletions or duplications that result in loss of dystrophin protein expression. There is currently no cure for DMD and the few treatment options available aim at slowing muscle degradation. New advances in gene therapy and understanding of dystrophin (DYS) expression in other muscular dystrophies have opened new opportunities for treatment. Therefore, reliable methods are needed to monitor dystrophin expression and assess the efficacy of new therapies for muscular dystrophies such as DMD and Becker muscular dystrophy (BMD). Here, we describe the validation of a novel Western blot (WB) method for the quantitation of mini-dystrophin protein in human skeletal muscle tissues that is easy to adopt in most laboratory settings. This WB method was assessed through precision, accuracy, selectivity, dilution linearity, stability, and repeatability. Based on mini-DYS standard performance, the assay has a dynamic range of 0.5-15 ng protein (per 5 µg total protein per lane), precision of 3.3 to 25.5%, and accuracy of - 7.5 to 3.3%. Our stability assessment showed that the protein is stable after 4 F/T cycles, up to 2 h at RT and after 7 months at - 70°C. Furthermore, our WB method was compared to the results from our recently published LC-MS method. Workflow for our quantitative WB method to determine mini-dystrophin levels in muscle tissues (created in Biorender.com). Step 1 involves protein extraction from skeletal muscle tissue lysates from control, DMD, or BMD biospecimen. Step 2 measures total protein concentrations. Step 3 involves running gel electrophoresis with wild-type dystrophin (wt-DYS) from muscle tissue extracts alongside mini-dystrophin STD curve and mini-DYS and protein normalization with housekeeping GAPDH.
Keywords: AAV9; Duchenne muscular dystrophy; Gene therapy; Quantitative; Western blot.
© 2022. The Author(s).
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


References
-
- Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687–95. - PubMed
-
- Association MD. Duchenne muscular dystrophy (DMD) 2021. [Available from: https://www.mda.org/disease/duchenne-muscular-dystrophy.
-
- Carter JC, Sheehan DW, Prochoroff A, Birnkrant DJ. Muscular dystrophies. Clin Chest Med. 2018;39(2):377–89. - PubMed
-
- Shieh PB. Muscular dystrophies and other genetic myopathies. Neurol Clin. 2013;31(4):1009–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

