Nationwide survey of refractory asthma with bronchiectasis by inflammatory subtypes
- PMID: 36539765
- PMCID: PMC9763800
- DOI: 10.1186/s12931-022-02289-y
Nationwide survey of refractory asthma with bronchiectasis by inflammatory subtypes
Abstract
Rationale: Bronchiectasis and bronchiolitis are differential diagnoses of asthma; moreover, they are factors associated with worse asthma control.
Objective: We determined clinical courses of bronchiectasis/bronchiolitis-complicated asthma by inflammatory subtypes as well as factors affecting them.
Methods: We conducted a survey of refractory asthma with non-cystic fibrosis bronchiectasis/bronchiolitis in Japan. Cases were classified into three groups, based on the latest fractional exhaled NO (FeNO) level (32 ppb for the threshold) and blood eosinophil counts (320/µL for the threshold): high (type 2-high) or low (type 2-low) FeNO and eosinophil and high FeNO or eosinophil (type 2-intermediate). Clinical courses in groups and factors affecting them were analysed.
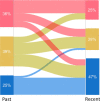
Results: In total, 216 cases from 81 facilities were reported, and 142 were stratified: 34, 40 and 68 into the type 2-high, -intermediate and -low groups, respectively. The frequency of bronchopneumonia and exacerbations requiring antibiotics and gram-negative bacteria detection rates were highest in the type 2-low group. Eighty-seven cases had paired latest and oldest available data of FeNO and eosinophil counts; they were analysed for inflammatory transition patterns. Among former type 2-high and -intermediate groups, 32% had recently transitioned to the -low group, to which relatively low FeNO in the past and oral corticosteroid use contributed. Lastly, in cases treated with moderate to high doses of inhaled corticosteroids, the frequencies of exacerbations requiring antibiotics were found to be higher in cases with more severe airway lesions and lower FeNO.
Conclusions: Bronchiectasis/bronchiolitis-complicated refractory asthma is heterogeneous. In patients with sputum symptoms and low FeNO, airway colonisation of pathogenic bacteria and infectious episodes are common; thus, corticosteroids should be carefully used.
Keywords: Asthma; Blood eosinophil counts; Bronchiectasis; Bronchiolitis; FeNO.
© 2022. The Author(s).
Conflict of interest statement
HM received lecturer fees from Novartis Japan, Sanofi K.K., AstraZeneca K.K., GlaxoSmithKline, Kyorin Pharmaceutical Co. and Boehringer Ingelheim received grants from Kyorin Pharmaceutical Co., Boehringer Ingelheim and Teijin Pharma outside the submitted work as well as received support from the Japanese Respiratory Society and Research Grant from Novartis Japan. AY received lecturer fees from AstraZeneca K.K., Novartis Japan, GlaxoSmithKline, Sanofi K.K. and Boehringer Ingelheim. YN received lecturer fees from Novartis Japan, AstraZeneca K.K. and Kyorin Pharmaceutical Co. KA received lecturer fees from Novartis Japan, Sanofi K.K., AstraZeneca K.K., GlaxoSmithKline plc and Boehringer Ingelheim Japan Inc. AN received lecturer fees from AstraZeneca K.K., Kyorin Pharmaceutical Co. and Novartis and received consulting fees from MSD. YT received lecturer fees from Kyorin Pharmaceutical Co., Teijin Pharma, Novartis Japan, Sanofi and AstraZeneca, received consulting fees from GlaxoSmithKline and Kyorin Pharmaceutical Co., and received grants from Kyorin Pharmaceutical Co., Astellas, Taiho, Boehringer Ingelheim and Teijin Pharma. NHar received lecturer fees from AstraZeneca K.K., GlaxoSmithKline K.K., Novartis Pharma K. K. and Sanofi K.K. and received grants from AstraZeneca K.K., Kyorin Pharmaceutical Co., Daikin (China) Investment Co., Ltd., Kao Corporation, SRL Medisearch Inc. and TOSOH Corporation. MN received lecturer fees from AstraZeneca K.K., GlaxoSmithKline K.K. and Torii Pharmaceutical Co. Ltd. HI received lecturer fees and/or advisory board from Astellas, AstraZeneca, Boehringer-Ingelheim, Fukuda-Denshi, GlaxoSmithKline, Kracie, Kyorin, Novartis, Omron, Pfizer and Sanofi and received research grants and support to Kagoshima University from Asahi-Kasei Pharma, AstraZeneca, Boehringer-Ingelheim, Chugai, GlaxoSmithKline, Kyorin, Otsuka, Teijin, Taiho and Ono. NM received lecturer fees from AstraZeneca K.K., GlaxoSmithKline K.K., Novartis Pharma K.K. and Nippon Boehringer Ingelheim Co., Ltd. Nhi received lecturer fees from AstraZeneca, GlaxoSmithKline and Novartis and received grants from GlaxoSmithKline and Novartis. MH received lecturer fees from AstraZeneca, GlaxoSmithKline and Novartis Pharma. Nhat received lecturer fees from AstraZeneca, GlaxoSmithKline, Novartis Pharma and Sanofi. Nhas received lecturer fees from AstraZeneca, GlaxoSmithKline, Novartis and Boehringer Ingelheim and received a research grant from Boehringer Ingelheim outside the submitted work. TK received lecturer fees from GlaxoSmithKline plc., Sanofi K.K., Nippon Boehringer Ingelheim Co., Ltd., Astra Zeneca K.K, Eli Lilly Japan K.K, Chugai Pharmaceutical Co., Ltd., Novartis Pharma K.K., Meiji Seika Pharma Co., Ltd., Bristol Myers Squibb, DAIICHI SANKYO COMPANY, LIMITED and TEIJIN PHARMA LIMITED. OM received lecturer fees from AstraZeneca, GlaxoSmithKline, Novartis Pharma and Sanofi. TO received lecturer fees from AstraZeneca K.K., GlaxoSmithKline plc., Boehringer Ingelheim Japan Inc., Kyorin Pharmaceutical Co., Novartis Japan and Sanofi K.K. HS belongs to the endowed chair supported by Phillips-Respironics, ResMed, Fukuda Denshi and Fukuda Lifetec Keiji.
Figures




References
-
- Padilla-Galo A, Olveira C, Fernández de Rota-Garcia L, Marco-Galve I, Plata AJ, Alvarez A, Rivas-Ruiz F, Carmona-Olveira A, Cebrian-Gallardo JJ, Martinez-Garcia MA. Factors associated with bronchiectasis in patients with uncontrolled asthma; the NOPES score: a study in 398 patients. Respir Res. 2018;19:43. doi: 10.1186/s12931-018-0746-7. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

