Underrepresentation of women in randomized controlled trials: a systematic review and meta-analysis
- PMID: 36539814
- PMCID: PMC9768985
- DOI: 10.1186/s13063-022-07004-2
Underrepresentation of women in randomized controlled trials: a systematic review and meta-analysis
Abstract
Background: Although regulatory changes towards correcting the underrepresentation of women in randomized controlled trials (RCTs) occurred (National Institutes of Health 1994), concerns exist about whether an improvement is taking place. In this systematic review and meta-analysis, we aimed to assess the inclusion rates of women in recent RCTs and to explore the potential barriers for the enrollment of women.
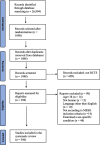
Methods: RCTs published in 2017 examining any type of intervention in adults were searched in PubMed and Cochrane Library. The following predefined medical fields were included: cardiovascular diseases, neoplasms, endocrine system diseases, respiratory tract diseases, bacterial and fungal infections, viral diseases, digestive system diseases, and immune system diseases. Studies were screened independently by two reviewers, and an equal number of studies was randomly selected per calendric month. The primary outcome was the enrollment rate of women, calculated as the number of randomized women patients divided by the total number of randomized patients. Rates were weighted by their inverse variance; statistical significance was tested using general linear models (GLM).
Results: Out of 398 RCTs assessed for eligibility, 300 RCTs were included. The enrollment rate of women in all the examined fields was lower than 50%, except for immune system diseases [median enrollment rate of 68% (IQR 46 to 81)]. The overall median enrollment rate of women was 41% (IQR 27 to 54). The median enrollment rate of women decreased with older age of the trials' participants [mean age of trials' participants ≤ 45 years: 47% (IQR 30-64), 46-55 years: 46% (IQR 33-58), 56-62 years: 38% (IQR 27-50), ≥ 63 years: 33% (IQR 20-46), p < 0.001]. Methodological quality characteristics showed no significant association with the enrollment rates of women. Out of the 300 included RCTs, eleven did not report on the number of included women. There was no significant difference between these studies and the studies included in the analysis.
Conclusions: Women are being inadequately represented, in the selected medical fields analyzed in our study, in recent RCTs. Older age is a potential barrier for the enrollment of women in clinical trials. Low inclusion rates of elderly women might create a lack of crucial knowledge in the adverse effects and the benefit/risk profile of any given treatment. Factors that might hinder the participation of women should be sought and addressed in the design of the study.
Keywords: Population external validity; Randomized controlled trials; Underrepresentation of women.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- Bracht GH, Glass GV. The external validity of experiments. Am Educ Res J. 1968;5(4):437–474. doi: 10.3102/00028312005004437. - DOI
-
- Institute of Medicine . Exploring the biological contributions to human health: does sex matter? Washington, DC: The National Academies Press; 2001. - PubMed
-
- National Institutes of Health. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. Federal Register. March 1994.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical