T cell autoimmunity and immune regulation to desmoglein 3, a pemphigus autoantigen
- PMID: 36539957
- PMCID: PMC10107879
- DOI: 10.1111/1346-8138.16663
T cell autoimmunity and immune regulation to desmoglein 3, a pemphigus autoantigen
Abstract
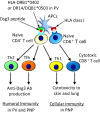
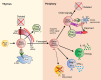
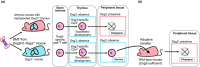
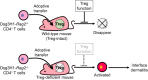
Pemphigus is a life-threatening autoimmune bullous disease mediated by anti-desmoglein IgG autoantibodies. Pemphigus is mainly classified into three subtypes: pemphigus vulgaris, pemphigus foliaceus, and paraneoplastic pemphigus. The pathogenicity of autoantibodies has been extensively studied. Anti-human CD20 antibody therapy targeting B cells emerged as a more effective treatment option compared to conventional therapy for patients with an intractable disease. On the other hand, autoreactive T cells are considered to be involved in the pathogenesis based on the test results of human leukocyte antigen association, autoreactive T cell detection, and cytokine profile analysis. Research on the role of T cells in pemphigus has continued to progress, including that on T follicular helper cells, which initiate molecular mechanisms involved in antibody production in B cells. Autoreactive T cell research in mice has highlighted the crucial roles of cellular autoimmunity and improved the understanding of its pathogenesis, especially in paraneoplastic pemphigus. The mouse research has helped elucidate novel regulatory mechanisms of autoreactive T cells, such as thymic tolerance to desmoglein 3 and the essential roles of regulatory T cells, Langerhans cells, and other molecules in peripheral tissues. This review focuses on the immunological aspects of autoreactive T cells in pemphigus by providing detailed information on various related topics.
Keywords: T cells; desmoglein; human leukocyte antigen; pemphigus; tolerance.
© 2022 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Pemphigus: a Comprehensive Review on Pathogenesis, Clinical Presentation and Novel Therapeutic Approaches.Clin Rev Allergy Immunol. 2018 Feb;54(1):1-25. doi: 10.1007/s12016-017-8662-z. Clin Rev Allergy Immunol. 2018. PMID: 29313220 Review.
-
Desmoglein 3-Reactive B Cells "Hiding" in Pemphigus Lesions.J Invest Dermatol. 2017 Nov;137(11):2255-2257. doi: 10.1016/j.jid.2017.07.823. J Invest Dermatol. 2017. PMID: 29055412
-
Immunophenotyping in pemphigus reveals a TH17/TFH17 cell-dominated immune response promoting desmoglein1/3-specific autoantibody production.J Allergy Clin Immunol. 2021 Jun;147(6):2358-2369. doi: 10.1016/j.jaci.2020.11.008. Epub 2020 Nov 20. J Allergy Clin Immunol. 2021. PMID: 33221382
-
Autoreactive T cells in the immune pathogenesis of pemphigus vulgaris.Exp Dermatol. 2013 Nov;22(11):699-704. doi: 10.1111/exd.12229. Exp Dermatol. 2013. PMID: 24433179 Review.
-
Pemphigus.Nat Rev Dis Primers. 2017 May 11;3:17026. doi: 10.1038/nrdp.2017.26. Nat Rev Dis Primers. 2017. PMID: 28492232 Free PMC article. Review.
Cited by
-
Disruption of post-thymic tolerance in skin-reactive TCR transgenic mice through the interaction of lymphopenia and intestinal microbiota.Int Immunol. 2024 Jul 13;36(8):413-424. doi: 10.1093/intimm/dxae018. Int Immunol. 2024. PMID: 38576231 Free PMC article.
-
Comparative Transcriptome Analysis Identifies Desmoglein-3 as a Potential Oncogene in Oral Cancer Cells.Cells. 2023 Nov 26;12(23):2710. doi: 10.3390/cells12232710. Cells. 2023. PMID: 38067138 Free PMC article.
-
Gut Microbiome Dysbiosis in Patients with Pemphigus and Correlation with Pathogenic Autoantibodies.Biomolecules. 2024 Jul 22;14(7):880. doi: 10.3390/biom14070880. Biomolecules. 2024. PMID: 39062594 Free PMC article.
-
Autoimmune diseases: targets, biology, and drug discovery.Acta Pharmacol Sin. 2024 Apr;45(4):674-685. doi: 10.1038/s41401-023-01207-2. Epub 2023 Dec 14. Acta Pharmacol Sin. 2024. PMID: 38097717 Free PMC article. Review.
-
Pemphigus Vulgaris Unmasked in a Patient With Behçet's Disease: A Complex Diagnostic Dilemma.Cureus. 2024 Sep 26;16(9):e70253. doi: 10.7759/cureus.70253. eCollection 2024 Sep. Cureus. 2024. PMID: 39463588 Free PMC article.
References
-
- Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded‐skin syndrome. N Engl J Med. 2006;355:1800–10. - PubMed
-
- Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti‐desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40:167–70. - PubMed
-
- Amagai M. Desmoglein as a target in autoimmunity and infection. J Am Acad Dermatol. 2003;48:244–52. - PubMed
-
- Anhalt GJ. Paraneoplastic pemphigus. Adv Dermatol. 1997;12:77–96; discussion 97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

