Bioactive 2-pyridone-containing heterocycle syntheses using multicomponent reactions
- PMID: 36540221
- PMCID: PMC9727751
- DOI: 10.1039/d2ra07056a
Bioactive 2-pyridone-containing heterocycle syntheses using multicomponent reactions
Abstract
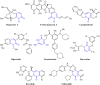
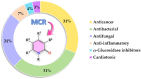
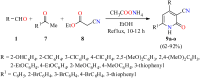
2-Pyridone-containing heterocycles are considered privileged scaffolds in drug discovery due to their behavior as hydrogen bond donors and/or acceptors and nonpeptidic mimics, and remarkable physicochemical properties such as metabolic stability, solubility in water, and lipophilicity. This review provides a comprehensive overview of multicomponent reactions (MCRs) for the synthesis of 2-pyridone-containing heterocycles. In particular, it covers the articles published from 1999 to date related to anticancer, antibacterial, antifungal, anti-inflammatory, α-glucosidase inhibitor, and cardiotonic activities of 2-pyridone-containing heterocycles obtained exclusively by an MCR. The discussion focuses on bioactivity data, synthetic approaches, plausible reaction mechanisms, and molecular docking simulations to facilitate comparison and underscore the applications of the 2-pyridone motif in drug discovery and medicinal chemistry. We also present our conclusions and outlook for the future.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
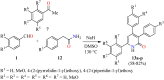
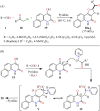
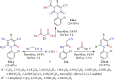
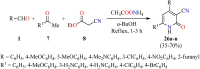
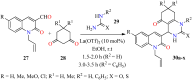
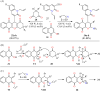
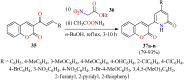
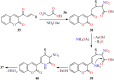

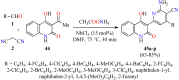
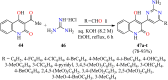
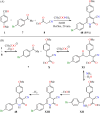
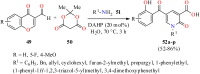


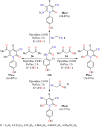
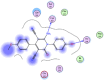
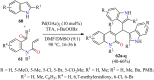
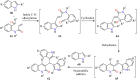
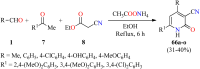





References
-
- Kakuchi R. Multicomponent reactions in polymer synthesis. Angew. Chem., Int. Ed. 2014;53:46–48. - PubMed
-
- Abonia R. Castillo J. Insuasty B. Quiroga J. Nogueras M. Cobo J. Efficient catalyst-free four-component synthesis of novel γ-aminoethers mediated by a Mannich type reaction. ACS Comb. Sci. 2013;15:2–9. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

