DFT and COSMO-RS studies on dicationic ionic liquids (DILs) as potential candidates for CO2 capture: the effects of alkyl side chain length and symmetry in cations
- PMID: 36540232
- PMCID: PMC9733716
- DOI: 10.1039/d2ra05805g
DFT and COSMO-RS studies on dicationic ionic liquids (DILs) as potential candidates for CO2 capture: the effects of alkyl side chain length and symmetry in cations
Abstract
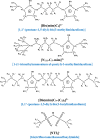
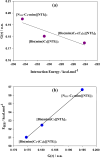
A few studies on CO2 capture using dicationic ionic liquids (DILs) show that they are more promising absorbents for CO2 capture than monocationic ILs (MILs). Ion-ion, ion-CO2 and DIL molecule-CO2 interactions are important for understanding the performance-structure-property relationships for the rational design of DILs for CO2 capture applications. However, the role of these interactions in determining CO2 solubility in DILs is unclear. In this study, we used DFT methods to understand these interactions in three selected DILs)considering the effects of alkyl side chain length and symmetry in cations (by exploring different aspects, such as the electronic and geometrical structures, topological properties and the strength and nature of interactions, charge transfer, etc. The results showed that the most suitable solvent for CO2 is the symmetric DIL with a longer side chain length, i.e. [Bis(mim)C5-(C4)2][NTf2]2. In addition, we used the COSMO-RS calculations to obtain the macroscopic solubility of CO2 in the studied DILs, which was in good agreement with the DFT results. Gas selectivity results calculated using COSMO-RS theory indicated that the selectivity of CO2 from H2, CO and CH4 gases decreases slightly with increasing the length of side alkyl chains.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Trickett C. A. Helal A. Al-Maythalony B. A. Yamani Z. H. Cordova K. E. Yaghi O. M. Nat. Rev. Mater. 2017;2:1–16.
-
- Sreedhar I. Nahar T. Venugopal A. Srinivas B. Renewable Sustainable Energy Rev. 2017;76:1080–1107.
-
- Dai Z. Noble R. D. Gin D. L. Zhang X. Deng L. J. Membr. Sci. 2016;497:1–20.
-
- Gurkan B. Simeon F. Hatton T. A. ACS Sustainable Chem. Eng. 2015;3:1394–1405.
-
- Masiren E. Harun N. Ibrahim W. Adam F. Int. J. Eng. Sci. Res. Technol. 2016;3:43–51.
LinkOut - more resources
Full Text Sources
Miscellaneous

