Lessons from SENCOVAC: a prospective study evaluating the response to SARS-CoV-2 vaccination in the CKD spectrum
- PMID: 36540904
- PMCID: PMC9756643
- DOI: 10.1016/j.nefro.2022.12.006
Lessons from SENCOVAC: a prospective study evaluating the response to SARS-CoV-2 vaccination in the CKD spectrum
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has negatively impacted on patients of the whole CKD spectrum, causing high rates of morbi-mortality. SARS-CoV-2 vaccines opened a new era, but patients with CKD (including kidney transplant, hemodialysis and peritoneal dialysis) were systematically excluded from pivotal clinical trials. The Spanish Society of Nephrology promoted the multicentric national SENCOVAC study aimed at assessing immunological responses after vaccination in patients with CKD. During the first year after vaccination, patients with non-dialysis CKD and those on hemodialysis and peritoneal dialysis presented good anti-Spike antibody responses to vaccination, especially after receiving the third and fourth doses. However, kidney transplant recipients presented suboptimal responses after any vaccination schedule (initial, third and fourth dose). Especially worrisome is the situation of a patients with a persistently negative humoral response that do not seroconvert after boosters. In this regard, monoclonal antibodies targeting SARS-CoV-2 have been approved for high-risk patients, although they may become obsolete as the viral genome evolves. The present report reviews the current status of SARS-CoV-2 vaccination in the CKD spectrum with emphasis on lessons learned from the SENCOVAC study. Predictors of humoral response, including vaccination schedules and types of vaccines, as well as the integration of vaccines, monoclonal antibodies and antiviral agents are discussed.
Síndrome agudo respiratorio severo coronavirus 2 (SARS-CoV-2) ha impactado negativamente en todos los pacientes con enfermedad renal crónica (ERC), causando elevadas tasas de morbimortalidad. La vacunación frente a SARS-CoV-2 han abierto una nueva era, aunque precisamente los pacientes con ERC (incluyendo los portadores de un injerto renal y aquellos en programas de hemodiálisis y diálisis peritoneal) han sido sistemáticamente excluidos de los ensayos clínicos. La Sociedad Española de Nefrología (S.E.N.) promovió el estudio multicéntrico SENCOVAC para evaluar la respuesta inmunológica tras la vacunación en todo el espectro de la ERC. Un año después de haber recibido la pauta inicial de vacunación, los pacientes con ERC sin necesidad de diálisis, y aquellos en hemodiálisis y diálisis peritoneal, han presenado una adecuada respuesta humoral (monitorizada con el desarrollo de anticuerpos frente a la proteína Spike), especialmente después de recibir la tercera y la cuarta dosis. Sin embargo, los portadores de un injerto renal han presentado una constante respuesta subóptima en cualquier momento de la vacunación (dosis inicial, tercera y cuarta dosis). Especialmente preocupante es la situación de los pacientes con respuesta humoral persistentemente negativa que no seroconvierten incluso ni tras recibir las dosis de recuerdo o boosters. En ese contexto, el manejo probablemente pasa por el uso de anticuerpos monoclonales dirigidos frente a SARS-CoV-2 que han sido recientemente aprobados, asumiendo que pueden perder efectividad con el cambio del genoma viral. La presente revision tiene por objeto resumir y analizar la situación actual de la vacunación frente a SARS-CoV-2 en el espectro de la ERC enfatizando en los resultados del estudio SENCOVAC. Asimismo, a lo largo de la revisión se discuten los predictores de la respuesta a las diferentes dosis y tipos de vacunas y la integración de estas con los anticuerpos monoclonales y los agentes antivirales.
Figures

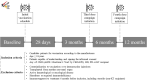

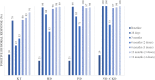
Cited by
-
COVID-19 and cardiovascular disease in patients with chronic kidney disease.Nephrol Dial Transplant. 2024 Jan 31;39(2):177-189. doi: 10.1093/ndt/gfad170. Nephrol Dial Transplant. 2024. PMID: 37771078 Free PMC article. Review.
-
Assessment of mRNA Vaccine Immunogenicity in Solid Organ Transplant Recipients.Medicina (Kaunas). 2023 Jun 2;59(6):1075. doi: 10.3390/medicina59061075. Medicina (Kaunas). 2023. PMID: 37374279 Free PMC article.
References
-
- Ortiz A. Asociación Información Enfermedades Renales Genéticas (AIRG-E), European Kidney Patients’ Federation (EKPF), Federación Nacional de Asociaciones para la Lucha Contra las Enfermedades del Riñón (ALCER), Fundación Renal Íñigo Álvarez de Toledo (FRIAT), Red de Investigación Renal (REDINREN), Resultados en Salud 2040 (RICORS2040), Sociedad Española de Nefrología (SENEFRO) Council, Sociedad Española de Trasplante (SET) Council, Organización Nacional de Trasplantes (ONT). RICORS2040: the need for collaborative research in chronic kidney disease. Clin Kidney J. 2022;15:372–387. doi: 10.1093/ckj/sfab170. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

