SARS-CoV-2 infection and vaccination in patients with hairy-cell leukaemia
- PMID: 36541031
- PMCID: PMC9877728
- DOI: 10.1111/bjh.18606
SARS-CoV-2 infection and vaccination in patients with hairy-cell leukaemia
Abstract
Little is known of the course of COVID-19 and the antibody response to infection or vaccination in patients with hairy-cell leukaemia (HCL). Among a total of 58 HCL cases we studied in these regards, 37 unvaccinated patients, mostly enjoying a relatively long period free from anti-leukaemic treatment, developed COVID-19 between March 2020 and December 2021 with a usually favourable outcome (fatality rate: 5/37, 14%); however, active leukaemia, older age and more comorbidities were associated with a worse course. Postinfection (n = 11 cases) and postvaccination (n = 28) seroconversion consistently developed, except after recent anti-CD20 or venetoclax therapy, correlating with perivaccine B-cell count. Vaccination appeared to protect from severe COVID-19 in 11 patients with breakthrough infection.
Keywords: COVID-19; anti-SARS-CoV-2 vaccination; hairy-cell leukaemia; post-COVID-19 seroconversion.
© 2022 British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest that are relevant to this report.
Figures
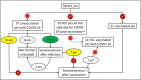
 , Vaccination against SARS‐CoV‐2;
, Vaccination against SARS‐CoV‐2;  , SARS‐CoV‐2 infection. *This vaccinated patient was tested for seroconversion before developing COVID‐19. ^These 10 patients were selected ad hoc for a history of COVID‐19 post vaccination (i.e., we cannot provide a denominator for this analysis), whereas the 11 vaccinated patients to the right were part of a systematic data collection effort (like the 37 unvaccinated COVID‐19‐positive patients to the left) and one of them (1/11) later developed COVID‐19. HCL, hairy‐cell leukaemia. Pt(s), patient(s).
, SARS‐CoV‐2 infection. *This vaccinated patient was tested for seroconversion before developing COVID‐19. ^These 10 patients were selected ad hoc for a history of COVID‐19 post vaccination (i.e., we cannot provide a denominator for this analysis), whereas the 11 vaccinated patients to the right were part of a systematic data collection effort (like the 37 unvaccinated COVID‐19‐positive patients to the left) and one of them (1/11) later developed COVID‐19. HCL, hairy‐cell leukaemia. Pt(s), patient(s).References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China. JAMA. 2020;323:1239–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

