Aged lipid-laden microglia display impaired responses to stroke
- PMID: 36541061
- PMCID: PMC9906381
- DOI: 10.15252/emmm.202217175
Aged lipid-laden microglia display impaired responses to stroke
Abstract
Microglial cells of the aged brain manifest signs of dysfunction that could contribute to the worse neurological outcome of stroke in the elderly. Treatment with colony-stimulating factor 1 receptor antagonists enables transient microglia depletion that is followed by microglia repopulation after treatment interruption, causing no known harm to mice. We tested whether this strategy restored microglia function and ameliorated stroke outcome in old mice. Cerebral ischemia/reperfusion induced innate immune responses in microglia highlighted by type I interferon and metabolic changes involving lipid droplet biogenesis. Old microglia accumulated lipids under steady state and displayed exacerbated innate immune responses to stroke. Microglia repopulation in old mice reduced lipid-laden microglia, and the cells exhibited reduced inflammatory responses to ischemia. Moreover, old mice with renewed microglia showed improved motor function 2 weeks after stroke. We conclude that lipid deposits in aged microglia impair the cellular responses to ischemia and worsen functional recovery in old mice.
Keywords: brain; immunometabolism; ischemia; lipid droplets; microglia.
© 2022 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

Representative P2YR12 immunostaining (green) of microglia of wild‐type male mice showing morphological differences between control microglia (a) and ischemic microglia (b–d). Ischemic microglial cells show typical phagocytic pouches (arrows). Nuclei are labeled with TO‐PRO‐3 (blue). Image (d) is a magnification of the area marked with a square in (c). Scale bar (a, b): 20 μm; (c): 10 μm; (d): 4 μm.
Transmission electron microscopy showing a microglial cell at the periphery of infarction 1 day postischemia surrounding remarkably swollen postsynaptic vesicles. Scale bar: 2 μm.
Microglia were obtained using fluorescence‐activated cell sorting (FACS) from the brain of control and ischemic young (3–4 months) male CX3CR1creERT2:Rosa26‐tdT mice 4 days postischemia (n = 4 mice per group). Microglia RNA was extracted for RNAseq analysis (GSE136856), as reported (Gallizioli et al, 2020).
Principal components analysis (PCA) shows sample distribution clearly separating microglia from control and ischemic mice.
Gene Ontology analysis illustrating pathways enriched in microglia after brain ischemia.
GSEA highlights the IFN‐α response pathway as highly upregulated after ischemia.
Genes of the Kegg pathway: Phagosomes are upregulated in microglia after ischemia. Color scale as in (F).
Most genes described as upregulated or downregulated in disease‐associated microglia (DAM) are accordingly regulated in microglia 4 days postischemia.

Expression of a selection of genes encoding lipid droplet‐associated proteins in microglia sorted from control (n = 4) and ischemic (n = 4) male mice at day four postischemia (obtained from RNAseq data shown in Fig 1). Heatmap illustrates ischemia‐induced upregulation of genes marked with * indicating adjusted P‐value < 0.001.
mRNA expression of PLIN2 and ISG15 in postmortem human brain tissue of nine stroke patients (points are values of individual patients); eight women (red points) and one man (black point). Samples were obtained from the ischemic tissue (Stroke) and nonaffected (NA) tissue. The graph shows boxes from the 25th to 75th percentiles, the median line, and whiskers down to the minimum and up to the maximum value, showing all points. Values are expressed as fold versus mean control (i.e., nonaffected tissue) Wilcoxon matched‐pairs signed‐rank test, **P = 0.0039; *P = 0.0195.
PLIN2 protein expression in mouse brain tissue, as assessed by Western blotting. Values were obtained from the ipsilateral hemisphere 1 h (n = 3), 4 h (n = 3), 15 h (n = 4), 24 h (n = 5) and 96 h (n = 5) postischemia, and 15 h (n = 4), 24 h (n = 2), and 96 h (n = 2) after sham operation. Values of sham mice were pooled together since they did not differ between time points. Samples of the contralateral hemisphere (Contra) of ischemic mice (1 h, n = 2; 24 h, n = 3; and 96 h, n = 3) were also evaluated. Ischemia increased PLIN2 expression at day 4 versus the sham group (**P = 0.0032, Kruskal–Wallis test and Dunn's multiple comparisons test). β‐tubulin is the protein loading control. The “Std” lane indicates the molecular weight standard. Quantification of band intensity where values are expressed as fold versus control (nonischemic). Points correspond to independent male mice and group values are expressed as a violin plot. Values were obtained 1 h (n = 3), 4 h (n = 3), 15 h (n = 4), 24 h (n = 5), and 96 h (n = 5) postischemia, and 15 h (n = 4), 24 h (n = 2), and 96 h (n = 2) after sham operation. Values of sham mice were pooled together since they did not differ between time points. Samples of the contralateral hemisphere (Contra) of ischemic mice (1 h, n = 2; 24 h, n = 3; and 96 h, n = 3) were also evaluated. Ischemia increased PLIN2 expression at day 4 (**P = 0.0032, Kruskal–Wallis test and Dunn's multiple comparisons test versus the sham group). Data are presented as violin plots with lines at the median and quartiles (dashed lines).
Immunofluorescence with antibodies against PLIN2 (green) and Iba‐1 (red) in brain tissue 1 day after induction of ischemia in female mice. Nuclei are stained with DAPI (blue). Control is the contralateral hemisphere. Scale bar: 10 μm.
Transmission electron microscopy showing microglial cells in control or ischemic tissue 1 day postischemia. Ischemia induces the formation of lipid droplets (LD) in microglia. LD are often seen near lysosomes (Lys). Insets in the lower panels are magnifications of the regions marked with a square. Scale bar 2 μm.
Flow cytometry gates to identify Bodipy+ microglia for control and ischemic brain tissue. Gates were set based on fluorescence minus one (FMO) intensity values.
Quantification of the percentage of CD11b+CD45low microglia containing lipid droplets as Bodipy+ microglia using flow cytometry in male mice deficient in Stat1 (Stat1−/−; n = 4) and corresponding Stat1+/+ mice (n = 7) shows ischemia‐induced increases in Stat1+/+ mice (**P = 0.0086) but not in Stat1−/− mice (P = 0.927; Two‐way ANOVA and Šídák's multiple comparisons test). The graph shows values of the nonischemic (−) and ischemic (+) brain hemispheres of individual mice and the mean ± SD.
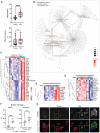
- A
Ischemia was induced in young and old female C57BL/6 mice (n = 17 per group) and infarct volume, as assessed with T2w MRI, and the neurological score was evaluated at day 4. The neurological score was higher (worse) in old mice (Mann–Whitney test, ****P < 0.0001), which also showed larger infarct volume (Mann–Whitney test, *P = 0.041) compared with young mice. The graph shows boxes from the 25th to 75th percentiles, the median line, and whiskers down to the minimum and up to the maximum value, showing all points.
- B
Microglia mRNA was obtained from young (3–4 months; n = 7) and old (21–22 months; n = 5) female mice 4 days postischemia and studied by RNAseq (GSE196737). Cnetplot illustrates the network of genes enriched after ischemia in old versus young mice linked to GO terms mainly related to innate immunity, inflammatory responses, and antigen presentation.
- C
Heatmaps of genes of representative innate immunity pathways enriched in microglia of old versus young ischemic mice.
- D, E
Upregulation of genes related to long‐chain fatty acid binding and downregulation of genes related to peroxisomal long‐chain fatty acid metabolism in microglia of old mice.
- F
Microglia obtained from young and old male (gray) and female (pink) mice 4 days postischemia (n = 16 mice; 4 mice per age group and sex) and sham operation (n = 12 mice; 3 mice per age group and sex) was studied by flow cytometry. After sham operation old mice showed a higher proportion of microglia containing lipid droplets after sham operation (***P < 0.001) and ischemia (**P = 0.0022; t‐test). Values show the mean ± SD.
- G
Microglia from adult young and old male mice using CD11b+ beads were kept in culture for 7 days and then exposed to red fluorescent pHrodo Escherichia coli bioparticles and stained with Bodipy. Bodipy+ lipid droplets (white in the upper panels ‐raw intensity‐ and green in the corresponding lower panels) are clearly seen in microglia from old mice, but not young mice. The images at the bottom also show phagocytosed red bioparticles, which are hardly seen in lipid droplets containing cells. The square in each image in the center is magnified in the images on the left and right sides for young and old microglia, respectively. Scale bar = 20 μm.

- A–C
Heatmaps illustrate genes upregulated in microglia of old ischemic mice versus young ischemic female mice for the following GO terms: “Response to IFN‐γ” (A), “Response to bacterium” (B), and “Antigen binding” (C).
- D
By contrast, downregulated pathways in microglia of old mice included: “β‐oxidation of very long‐chain fatty acids”.

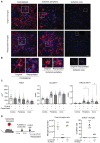
- A, B
(A) Representative images of the striatum of young female mice with original microglia and repopulated microglia 4 days postischemia (n = 4 per group). Images show microglial cells immunostained with anti‐P2YR12 (red) in the noninjured contralateral hemisphere, the periphery of ischemia, and the lesion core. Cell nuclei are labeled with DAPI (blue). After repopulation, original and renewed microglia react to ischemia with changes in morphology. (B) Magnification of individual cells marked with squares in (D). Scale bar: (A) 20 μm, (B) 10 μm.
- C
Morphometric analysis of microglial cells showed reduced area and increased solidity and circularity in microglia of the ischemic hemisphere versus the contralateral hemisphere in both groups. However, the increase in circularity at the periphery and core of infarction was attenuated in repopulated versus original microglia (*P = 0.047 in the periphery and *P = 0.033 in the core; Kruskal–Wallis test). Points show individual cells (in the periphery, core, and contralateral regions: n = 233, 96, and 91 cells for the control group, and n = 178, 58, and 109 cells for the renewed group, respectively), and colors indicate different mice (n = 4 mice per group). Bars show the median with 95% confidence interval.
- D
Renewed microglia derive from brain cells. We generated chimeric mice by bone marrow transplantation from DsRed fluorescent reporter donor male mice to wild‐type recipient male mice (n = 11). After at least 8 weeks, mice were treated with PLX5622 in the diet (n = 8) or corresponding control diet (n = 3). Three weeks later, mice were euthanized (n = 4) or they were switched to control diet for repopulation for 7 days (n = 4 per group). The brain was studied via flow cytometry by measuring DsRed+ and DsRed− cells in the gate of microglia cells. Absolute number of microglial cells was strongly reduced after microglia depletion (PLX5622 diet; one‐way ANOVA and Holm–Šídák's multiple comparisons test ***P = 0.0002 versus control diet). Microglia numbers recovered after 7 days of repopulation (&&& P = 0.0002 versus depleted cells). The % of DsRed cells in the microglia gate of mice fed control diet was negligible. However, after microglia depletion (PLX5622 treatment; ANOVA and Holm–Šídák's, ***P < 0.0001 versus control diet) there was a high % of DsRed+ cells within the very small population of CD45lowCD11b+ cells indicating the presence of a few CSF1R‐independent infiltrating cells. Importantly, the proportion of DsRed+ cells was negligible after mice were switched to control diet and the number of microglia increased (&&& P < 0.0001 versus depleted mice). Values are expressed as the mean ± SEM.

- A
Experimental design for microglia depletion and repopulation in young (3–4 months; n = 39) and old (20–22 months; n = 34) female mice. We depleted microglia via a PLX5622 diet for 3 weeks. Mice were repopulated by switching to the corresponding control diet for 3, 7, or 21 days prior to ischemia induction. The brain was studied 4 days postischemia and during this period control diet was maintained. Total repopulation times: 7, 11, and 25 days, respectively. As controls, we used mice subjected to control diet and studied 4 days postischemia or sham operation.
- B
The number of microglia recovered postischemia was lower in old (O) than in young (Y) mice fed a control diet (two‐way ANOVA and Šídák's multiple comparisons test, #P = 0.0134). The PLX5622 diet strongly reduced the number of microglial cells postischemia in both age groups (&&& P < 0.0001 versus ischemic mice on control diet); switching to control diet increased the number of microglia versus depleted mice (***P < 0.0001 at day 7, ***P = 0.0003 at day 11, and *P = 0.0377 at day 25; two‐way ANOVA and Dunnett's multiple comparison test). Ischemic mice: n = 10 Y and n = 9 O with control diet; n = 7 Y and n = 4 O with PLX5622 diet (depleted); n = 5 Y and n = 5 O with PLX5622 diet + 7 d (repopulated for 7 days); n = 4 Y and n = 8 O with PLX5622 diet + 11 d (repopulated for 11 days); n = 2 Y and n = 3 O with PLX5622 diet + 25 d (repopulated for 25 days). Sham mice: n = 8 Y and n = 5 O.
- C
Gating strategy for cell sorting to obtain the CD45lowCD11b+ microglia shown in (B).

Young (3–4 months) and old (21–22 months) female mice were fed control diet (n = 12; n = 5 old mice and n = 7 young mice) or were treated with diet containing PLX5622 for 21 days to deplete microglia followed by restoration of control diet for 7 days to induce microglia repopulation (n = 11; n = 8 old mice and n = 3 young mice). Microglia were FACS sorted and RNA extracted for comparison of the transcriptomic profile of microglia obtained from old female ischemic mice with repopulated microglia (GSE196737). Created with BioRender.
Cnetplot illustrating GO networks of DEGs in renewed microglia versus original microglia of old mice after brain ischemia.
Heatmap showing reduced expression of genes related to the GO term “Response to virus” in renewed microglia.
Venn diagram showing DEGs common and unique to the comparisons of the microglia obtained under the various experimental conditions after ischemia in female mice. There are 137 DEGs common for “microglia of old versus young ischemic mice” and “repopulated (re) versus original microglia of old ischemic mice” (upper diagram). Notably, none of these 137 genes is commonly upregulated or downregulated, signifying that they go in opposite directions in each comparison.
Heatmap illustrating expression of 75 annotated genes of this group. Microglia renewal prevents the effect of old age by upregulating the expression of genes previously downregulated in old microglia and vice versa.
Cnetplot illustrating the results of pathway analysis of the aforementioned 75 genes shows that the most enriched pathways correspond to IFN signaling.

- A–E
Heatmaps illustrate DEGs in various GO pathways downregulated (A–C) and upregulated (D, E) in renewed microglia of old mice after ischemia in relation to the original microglia of old ischemic mice. Downregulated GO terms include: (A) “Response to type I IFN,” (B) “Pyroptosis,” and (C) “Antigen binding.” By contrast, enriched pathways in renewed microglia include: (D) “Mitochondrial respirasome” and (E) “Fatty acid metabolism”.

Heatmap of lipid droplet‐associated proteins in microglia of young (n = 7) and old (n = 5) female mice and corresponding groups with microglia renewal (Re) under ischemia (n = 3 and n = 8). Old microglia show increased expression of some genes versus young microglia (& P < 0.05). Microglia renewal in old mice prevents the age‐dependent increase in type I IFN genes (# P < 0.05).
Box plots depict normalized gene expression of representative genes encoding lipid droplet‐associated proteins of the IFN pathway. The graph shows boxes from the 25th to 75th percentiles, the median line, and whiskers down to the minimum and up to the maximum value, showing all points.
Representative flow cytometry plots showing Bodipy+ CD11b+CD45low microglia from old male mice with or without microglia renewal. Data show results from the ischemic brain hemisphere.
Quantification of flow cytometry shows that microglia renewal reduced the percentage of Bodipy+ microglia in old mice in sham (***P = 0.0001) and ischemic (*P = 0.0057) conditions (one‐way ANOVA and Holm–Šídák's multiple comparisons test). For illustrative purposes, the values of young mice are also shown (original old versus young: ***P < 0.001 in the sham group and **P = 0.0026 in the ischemic group). Values show individual mice and bars show the mean ± SD.
Primary microglia cultures were obtained using CD11b+ magnetic beads from old male mice with or without microglia renewal (n = 2 per group). Cells were exposed to red fluorescent pHrodo Escherichia coli bioparticles and were stained with green fluorescent Bodipy and studied by confocal microscopy. Black and white images illustrate raw intensity data of Bodipy staining. The lower images are corresponding merged channels showing Bodipy in green, phagocytosed bioparticles in red, and the DAPI+ nuclei in blue. Repopulated microglia show less lipid droplets and the cytoplasm is replenished with phagocytosed bioparticles. The square regions of the images indicate areas magnified at the side of each image. Scale bar = 20 μm.
Electron microscopy images representative of microglia of old male mice with original microglia (Old) and with renewed microglia (Old‐Renewed; n = 3 per group). Images labeled (a–d) are higher magnifications of the areas marked with yellow squares in the images on the left. Lipid droplets are marked with arrows. Large lipid droplets are seen in microglia of old mice, whereas renewed microglia typically show no or small lipid droplets.

Experimental design for microglia depletion and repopulation in old (21–22 month) female mice. We depleted microglia by providing a diet containing the CSF1R antagonist PLX5622 for 3 weeks. Microglia repopulation was induced by switching to control diet for 7 days prior to induction of ischemia (n = 17). Treatment controls were fed a corresponding control diet (n = 16). Behavioral testing (including training sessions) was conducted throughout the experiment and mice underwent two MRI studies. Measures were obtained the day prior to ischemia (basal value, time = 0) and at different time points postischemia until day 14. Image created with BioRender.
The neuroscore value (mean ± SD) decreased from day 1 to day 14 by 23% in the control group (from 10.8 ± 4.7 to 8.3 ± 4.2; n = 15) and by 46% in the microglia renewed group (from 11.9 ± 6.1 to 6.4 ± 2.8; n = 16). The variability within groups was large and differences between groups were not statistically significant (P = 0.21, nonparametric Kruskal–Wallis test with a repeated‐measures design). Points are individual values for each mouse, box and whiskers show the median, and bars indicate min. and max. values. One animal of each group died after ischemia and these mice were excluded. The grip test assessing limb strength showed a better performance in the group with repopulated microglia (Two‐way ANOVA and Šídák's multiple comparisons test, *P = 0.047). Values are expressed as % of the corresponding basal value (preischemia) for each mouse. Rotarod test did not show differences between groups. Values are expressed as latency to fall (seconds) from the running wheel at different time points postischemia (higher means better). Laterality was assessed via the cylinder test in a subgroup of mice (n = 7 renewed, n = 6 control). Positive values in the laterality index (ranging from −1 to +1) indicate impairment of the affected hindlimb, i.e., contralateral to the injured brain hemisphere. There were no differences between groups with the Mann–Whitney test. However, data analysis with the Wilcoxon signed‐rank test showed that the repopulated group was significantly different (*P = 0.016) from a theoretical median of +1 at day 4 postischemia whereas the control group was not (P = 0.062). The results indicate some transient laterality effect in the control group at day 4 postischemia that was not detected in the repopulated group. Bars represent mean ± SD.
The MRI lesion volume, as illustrated in the corresponding false color images below, showed no differences in infarct volume in mice with (n = 16) or without (n = 15) microglia renewal. Results correspond to day four postischemia. Representative MRI images were obtained on day four postischemia using T2w MRI and day 14 using a Modified Driven Equilibrium Fourier Transform (MDEFT) MRI sequence (7.0 T BioSpec 70/30, Bruker BioSpin). Values show data for individual mice and/or the mean ± SD.
References
-
- Androvic P, Kirdajova D, Tureckova J, Zucha D, Rohlova E, Abaffy P, Kriska J, Valny M, Anderova M, Kubista M et al (2020) Decoding the transcriptional response to ischemic stroke in Young and aged mouse brain. Cell Rep 31: 107777 - PubMed
-
- Belhocine S, Machado Xavier A, Distéfano‐Gagné F, Fiola S, Rivest S, Gosselin D (2021) Context‐dependent transcriptional regulation of microglial proliferation. Glia 70: 572–589 - PubMed
-
- Bosch M, Sánchez‐Álvarez M, Fajardo A, Kapetanovic R, Steiner B, Dutra F, Moreira L, López JA, Campo R, Marí M et al (2020) Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science 370: eaay8085 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

