PEX13 prevents pexophagy by regulating ubiquitinated PEX5 and peroxisomal ROS
- PMID: 36541703
- PMCID: PMC10262789
- DOI: 10.1080/15548627.2022.2160566
PEX13 prevents pexophagy by regulating ubiquitinated PEX5 and peroxisomal ROS
Abstract
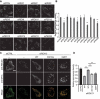
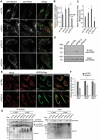
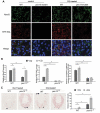
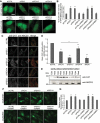
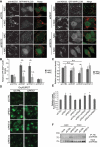
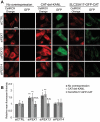
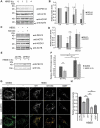
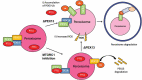
Peroxisomes are rapidly degraded during amino acid and oxygen deprivation by a type of selective autophagy called pexophagy. However, how damaged peroxisomes are detected and removed from the cell is poorly understood. Recent studies suggest that the peroxisomal matrix protein import machinery may serve double duty as a quality control machinery, where they are directly involved in activating pexophagy. Here, we explored whether any matrix import factors are required to prevent pexophagy, such that their loss designates peroxisomes for degradation. Using gene editing and quantitative fluorescence microscopy on culture cells and a zebrafish model system, we found that PEX13, a component of the peroxisomal matrix import system, is required to prevent the degradation of otherwise healthy peroxisomes. The loss of PEX13 caused an accumulation of ubiquitinated PEX5 on peroxisomes and an increase in peroxisome-dependent reactive oxygen species that coalesce to induce pexophagy. We also found that PEX13 protein level is downregulated to aid in the induction of pexophagy during amino acid starvation. Together, our study points to PEX13 as a novel pexophagy regulator that is modulated to maintain peroxisome homeostasis.Abbreviations: AAA ATPases: ATPases associated with diverse cellular activities; ABCD3: ATP binding cassette subfamily D member; 3ACOX1: acyl-CoA oxidase; 1ACTA1: actin alpha 1, skeletal muscle; ACTB: actin beta; ATG5: autophagy related 5; ATG7: autophagy related 7; ATG12: autophagy related 12; ATG16L1: autophagy related 16 like 1; CAT: catalase; CQ: chloroquine; Dpf: days post fertilization: FBS: fetal bovine serum; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFP: green fluorescent protein; H2O2: hydrogen peroxide; HA - human influenza hemagglutinin; HBSS: Hanks' Balanced Salt Solution; HCQ; hydroxychloroquine; KANL: lysine alanine asparagine leucine; KO: knockout; MAP1LC3B: microtubule associated protein 1 light chain 3 beta; MEF: mouse embryonic fibroblast; MTOR: mechanistic target of rapamycin kinase; MTORC1: mechanistic target of rapamycin kinase complex 1; MTORC2: mechanistic target of rapamycin kinase complex 2; MYC: MYC proto-oncogene, bHLH transcription factor; MZ: maternal and zygotic; NAC: N-acetyl cysteine; NBR1 - NBR1 autophagy cargo receptor; PBD: peroxisome biogenesis disorder; PBS: phosphate-buffered saline; PEX: peroxisomal biogenesis factor; PTS1: peroxisome targeting sequence 1; RFP: red fluorescent protein; ROS: reactive oxygen speciess; iRNA: short interfering RNA; SKL: serine lysine leucine; SLC25A17/PMP34: solute carrier family 25 member 17; Ub: ubiquitin; USP30: ubiquitin specific peptidase 30.
Keywords: PEX13; PEX5; Peroxisomes; pexophagy; reactive oxygen species; selective autophagy; ubiquitin; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Wanders RJA, Waterham HR.. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. 2006;75(1):295–332. http://www.annualreviews.org/doi/10.1146/annurev.biochem.74.082803.133329 - DOI - PubMed
-
- Farré J, Mahalingam SS, Proietto M, et al. Peroxisome biogenesis, membrane contact sites, and quality control. EMBO Rep. [Internet]. 2018;e46864. http://embor.embopress.org/lookup/doi/10.15252/embr.201846864 - DOI - PMC - PubMed
-
- Fidaleo M. Peroxisomes and peroxisomal disorders: the main facts.Exp Toxicol Pathol.2010;62(6):615–625. - PubMed
-
- Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. Internet]. 1972;128:617–630. http://www.ncbi.nlm.nih.gov/pubmed/4404507%0Ahttp://www.pubmedcentral.ni... - PMC - PubMed
-
- Waterham HR, Ferdinandusse S, Wanders RJA. Human disorders of peroxisome metabolism and biogenesis.Biochim Biophys Acta Mol Cell Res.2016;1863(5):922–933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
