Within or Without You? A Perspective Comparing In Situ and Ex Situ Tissue Engineering Strategies for Articular Cartilage Repair
- PMID: 36541723
- PMCID: PMC11468013
- DOI: 10.1002/adhm.202201305
Within or Without You? A Perspective Comparing In Situ and Ex Situ Tissue Engineering Strategies for Articular Cartilage Repair
Abstract
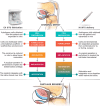
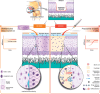
Human articular cartilage has a poor ability to self-repair, meaning small injuries often lead to osteoarthritis, a painful and debilitating condition which is a major contributor to the global burden of disease. Existing clinical strategies generally do not regenerate hyaline type cartilage, motivating research toward tissue engineering solutions. Prospective cartilage tissue engineering therapies can be placed into two broad categories: i) Ex situ strategies, where cartilage tissue constructs are engineered in the lab prior to implantation and ii) in situ strategies, where cells and/or a bioscaffold are delivered to the defect site to stimulate chondral repair directly. While commonalities exist between these two approaches, the core point of distinction-whether chondrogenesis primarily occurs "within" or "without" (outside) the body-can dictate many aspects of the treatment. This difference influences decisions around cell selection, the biomaterials formulation and the surgical implantation procedure, the processes of tissue integration and maturation, as well as, the prospects for regulatory clearance and clinical translation. Here, ex situ and in situ cartilage engineering strategies are compared: Highlighting their respective challenges, opportunities, and prospects on their translational pathways toward long term human cartilage repair.
Keywords: 3D bioprinting; biomaterials; cartilage tissue engineering; in situ tissue engineering; stem cells.
© 2022 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Chondroinductive Peptides for Cartilage Regeneration.Tissue Eng Part B Rev. 2022 Aug;28(4):745-765. doi: 10.1089/ten.TEB.2021.0125. Epub 2021 Oct 18. Tissue Eng Part B Rev. 2022. PMID: 34375146 Review.
-
Multiphasic, Multistructured and Hierarchical Strategies for Cartilage Regeneration.Adv Exp Med Biol. 2015;881:143-60. doi: 10.1007/978-3-319-22345-2_9. Adv Exp Med Biol. 2015. PMID: 26545749
-
Microenvironmentally optimized 3D-printed TGFβ-functionalized scaffolds facilitate endogenous cartilage regeneration in sheep.Acta Biomater. 2022 Sep 15;150:181-198. doi: 10.1016/j.actbio.2022.07.029. Epub 2022 Jul 25. Acta Biomater. 2022. PMID: 35896136
-
3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects.Acta Biomater. 2020 Sep 1;113:130-143. doi: 10.1016/j.actbio.2020.05.040. Epub 2020 Jun 4. Acta Biomater. 2020. PMID: 32505800
-
Endogenous Tissue Engineering for Chondral and Osteochondral Regeneration: Strategies and Mechanisms.ACS Biomater Sci Eng. 2024 Aug 12;10(8):4716-4739. doi: 10.1021/acsbiomaterials.4c00603. Epub 2024 Aug 2. ACS Biomater Sci Eng. 2024. PMID: 39091217 Review.
Cited by
-
A dynamically loaded ex vivo model to study neocartilage and integration in human cartilage repair.Front Cell Dev Biol. 2024 Sep 30;12:1449015. doi: 10.3389/fcell.2024.1449015. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39403130 Free PMC article.
-
Construction of 3D-Bioprinted cartilage-mimicking substitute based on photo-crosslinkable Wharton's jelly bioinks for full-thickness articular cartilage defect repair.Mater Today Bio. 2023 Jun 9;21:100695. doi: 10.1016/j.mtbio.2023.100695. eCollection 2023 Aug. Mater Today Bio. 2023. PMID: 37384040 Free PMC article.
-
Injectable and in situ foaming shape-adaptive porous Bio-based polyurethane scaffold used for cartilage regeneration.Bioact Mater. 2024 May 13;39:1-13. doi: 10.1016/j.bioactmat.2024.03.012. eCollection 2024 Sep. Bioact Mater. 2024. PMID: 38783924 Free PMC article.
-
Controlled oxygen delivery to power tissue regeneration.Nat Commun. 2024 May 22;15(1):4361. doi: 10.1038/s41467-024-48719-x. Nat Commun. 2024. PMID: 38778053 Free PMC article. Review.
-
Silk fibroin-based inks for in situ 3D printing using a double crosslinking process.Bioact Mater. 2024 Jan 25;35:122-134. doi: 10.1016/j.bioactmat.2024.01.015. eCollection 2024 May. Bioact Mater. 2024. PMID: 38312518 Free PMC article.
References
-
- Davies‐Tuck M. L., Wluka A. E., Wang Y., Teichtahl A. J., Jones G., Ding C., Cicuttini F. M., Osteoarthritis Cartilage 2008, 16, 337. - PubMed
-
- Kingsbury S. R., Gross H. J., Isherwood G., Conaghan P. G., Rheumatology 2014, 53, 937. - PubMed
-
- Australian Bureau of Statistics , National Health Survey: First Results 2017–18, Canberra, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

