In Vitro Activity of Cefepime-Taniborbactam and Comparators against Clinical Isolates of Gram-Negative Bacilli from 2018 to 2020: Results from the Global Evaluation of Antimicrobial Resistance via Surveillance (GEARS) Program
- PMID: 36541767
- PMCID: PMC9872668
- DOI: 10.1128/aac.01281-22
In Vitro Activity of Cefepime-Taniborbactam and Comparators against Clinical Isolates of Gram-Negative Bacilli from 2018 to 2020: Results from the Global Evaluation of Antimicrobial Resistance via Surveillance (GEARS) Program
Abstract
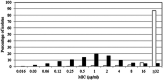
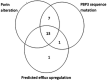
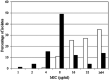
Taniborbactam is a novel cyclic boronate β-lactamase inhibitor in clinical development in combination with cefepime. We assessed the in vitro activity of cefepime-taniborbactam and comparators against a 2018-2020 collection of Enterobacterales (n = 13,731) and Pseudomonas aeruginosa (n = 4,619) isolates cultured from infected patients attending hospitals in 56 countries. MICs were determined by CLSI broth microdilution. Taniborbactam was tested at a fixed concentration of 4 μg/mL. Isolates with cefepime-taniborbactam MICs of ≥16 μg/mL underwent whole-genome sequencing. β-lactamase genes were identified in meropenem-resistant isolates by PCR/Sanger sequencing. Against Enterobacterales, taniborbactam reduced the cefepime MIC90 value by >64-fold (from >16 to 0.25 μg/mL). At ≤16 μg/mL, cefepime-taniborbactam inhibited 99.7% of all Enterobacterales isolates; >97% of isolates with multidrug-resistant (MDR) and ceftolozane-tazobactam-resistant phenotypes; ≥90% of isolates with meropenem-resistant, difficult-to-treat-resistant (DTR), meropenem-vaborbactam-resistant, and ceftazidime-avibactam-resistant phenotypes; 100% of VIM-positive, AmpC-positive, and KPC-positive isolates; 98.7% of extended-spectrum β-lactamase (ESBL)-positive; 98.8% of OXA-48-like-positive; and 84.6% of NDM-positive isolates. Against P. aeruginosa, taniborbactam reduced the cefepime MIC90 value by 4-fold (from 32 to 8 μg/mL). At ≤16 μg/mL, cefepime-taniborbactam inhibited 97.4% of all P. aeruginosa isolates; ≥85% of isolates with meropenem-resistant, MDR, and meropenem-vaborbactam-resistant phenotypes; >75% of isolates with DTR, ceftazidime-avibactam-resistant, and ceftolozane-tazobactam-resistant phenotypes; and 87.4% of VIM-positive isolates. Multiple potential mechanisms, including carriage of IMP, certain alterations in PBP3, permeability (porin) defects, and possibly, upregulation of efflux were present in most isolates with cefepime-taniborbactam MICs of ≥16 μg/mL. We conclude that cefepime-taniborbactam exhibited potent in vitro activity against Enterobacterales and P. aeruginosa and inhibited most carbapenem-resistant isolates, including those carrying serine carbapenemases or NDM/VIM metallo-β-lactamases (MBLs).
Keywords: Enterobacterales; Gram-negative; Pseudomonas aeruginosa; carbapenem resistant; multidrug resistant; taniborbactam; β-lactamase inhibitor.
Conflict of interest statement
The authors declare a conflict of interest. D.A.S., T.U., D.M.D., S.M.C., D.C.P., and G.M. are employees of Venatorx Pharmaceuticals, Inc. M.A.H., M.G.W., D.F.S., and J.A.K. do not have personal financial interests in the sponsor of the study.
Figures

References
-
- Castanheira M, Deshpande L, Mendes RE, Canton R, Sader HS, Jones RN. 2019. Variations in the occurrence of resistance phenotypes and carbapenemase genes among Enterobacteriaceae isolates in 20 years of the SENTRY antimicrobial surveillance program. Open Forum Infect Dis 6:S23–S33. doi: 10.1093/ofid/ofy347. - DOI - PMC - PubMed
-
- Kazmierczak KM, Karlowsky JA, de Jonge BLM, Stone GG, Sahm DF. 2021. Epidemiology of carbapenem resistance determinants identified in meropenem-nonsusceptible Enterobacterales collected as part of a global surveillance program, 2012 to 2017. Antimicrob Agents Chemother 65:e02000-20. doi: 10.1128/AAC.02000-20. - DOI - PMC - PubMed
-
- Kazmierczak KM, de Jonge BLM, Stone GG, Sahm DF. 2020. Longitudinal analysis of ESBL and carbapenemase carriage among Enterobacterales and Pseudomonas aeruginosa isolates collected in Europe as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance programme, 2013–2017. J Antimicrob Chemother 75:1165–1173. doi: 10.1093/jac/dkz571. - DOI - PubMed
-
- World Health Organization. 2017. WHO publishes list of bacteria for which new antibiotics are urgently needed. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

