Race and Ethnicity Reporting and Representation in Obstetrics and Gynecology Clinical Trials and Publications From 2007-2020
- PMID: 36542396
- PMCID: PMC9856739
- DOI: 10.1001/jamasurg.2022.6600
Race and Ethnicity Reporting and Representation in Obstetrics and Gynecology Clinical Trials and Publications From 2007-2020
Erratum in
-
Error in the Author Affiliations.JAMA Surg. 2023 Feb 1;158(2):224. doi: 10.1001/jamasurg.2022.8308. JAMA Surg. 2023. PMID: 36752802 Free PMC article. No abstract available.
Abstract
Importance: Clinical trials guide evidence-based obstetrics and gynecology (OB-GYN) but often enroll nonrepresentative participants.
Objective: To characterize race and ethnicity reporting and representation in US OB-GYN clinical trials and their subsequent publications and to analyze the association of subspecialty and funding with diverse representation.
Design and setting: Cross-sectional analysis of all OB-GYN studies registered on ClinicalTrials.gov (2007-2020) and publications from PubMed and Google Scholar (2007-2021). Analyses included logistic regression controlling for year, subspecialty, phase, funding, and site number. Data from 332 417 studies were downloaded. Studies with a noninterventional design, with a registration date before October 1, 2007, without relevance to OB-GYN, with no reported results, and with no US-based study site were excluded.
Exposures: OB-GYN subspecialty and funder.
Main outcomes and measures: Reporting of race and ethnicity data and racial and ethnic representation (the proportion of enrollees of American Indian or Alaskan Native, Asian, Black, Latinx, or White identity and odds of representation above US Census estimates by race and ethnicity).
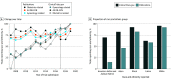
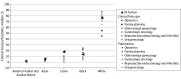
Results: Among trials with ClinicalTrials.gov results (1287 trials with 591 196 participants) and publications (1147 trials with 821 111 participants), 662 (50.9%) and 856 (74.6%) reported race and ethnicity data, respectively. Among publications, gynecology studies were significantly less likely to report race and ethnicity than obstetrics (adjusted odds ratio [aOR], 0.54; 95% CI, 0.38-0.75). Reproductive endocrinology and infertility trials had the lowest odds of reporting race and ethnicity (aOR, 0.14; 95% CI, 0.07-0.27; reference category, obstetrics). Obstetrics and family planning demonstrated the most diverse clinical trial cohorts. Compared with obstetric trials, gynecologic oncology had the lowest odds of Black representation (ClinicalTrials.gov: aOR, 0.04; 95% CI, 0.02-0.09; publications: aOR, 0.06; 95% CI, 0.03-0.11) and Latinx representation (ClinicalTrials.gov: aOR, 0.05; 95% CI, 0.02-0.14; publications: aOR, 0.23; 95% CI, 0.10-0.48), followed by urogynecology and reproductive endocrinology and infertility. Urogynecology (ClinicalTrials.gov: aOR, 0.15; 95% CI, 0.05-0.39; publications: aOR, 0.24; 95% CI, 0.09-0.58) had the lowest odds of Asian representation.
Conclusions and relevance: Race and ethnicity reporting and representation in OB-GYN trials are suboptimal. Obstetrics and family planning trials demonstrate improved representation is achievable. Nonetheless, all subspecialties should strive for more equitably representative research.
Conflict of interest statement
Figures


Comment in
-
Clinical Trial Reporting and Representation-An Opportunity to Advance Health Equity.JAMA Surg. 2023 Feb 1;158(2):190-191. doi: 10.1001/jamasurg.2022.6601. JAMA Surg. 2023. PMID: 36542364 No abstract available.
References
-
- Chen MS Jr, Lara PN, Dang JHT, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120(suppl 7):1091-1096. doi:10.1002/cncr.28575 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

