Effects of a Smartphone-Based Self-management Intervention for Individuals With Bipolar Disorder on Relapse, Symptom Burden, and Quality of Life: A Randomized Clinical Trial
- PMID: 36542401
- PMCID: PMC9857325
- DOI: 10.1001/jamapsychiatry.2022.4304
Effects of a Smartphone-Based Self-management Intervention for Individuals With Bipolar Disorder on Relapse, Symptom Burden, and Quality of Life: A Randomized Clinical Trial
Abstract
Importance: Bipolar disorder-specific psychotherapy combined with pharmacotherapy improves relapse risk, symptom burden, and quality of life, but psychotherapy is not easily accessible.
Objective: To determine if a smartphone-based self-management intervention (LiveWell) can assist individuals with bipolar disorder to maintain wellness.
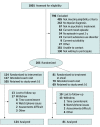
Design, setting, and participants: An assessor-blind randomized clinical trial enrolled participants from March 20, 2017, to April 25, 2019, with 48-week follow-up ending on April 10, 2020. Participants were randomly assigned to usual care or usual care plus the smartphone intervention stratified by relapse risk based on initial clinical status (low risk: asymptomatic recovery; high risk: continued symptomatic, prodromal, recovering, symptomatic recovery). Participants with bipolar disorder I were recruited from clinics in the Chicago and Minneapolis-Saint Paul areas. Data were analyzed from June 19, 2020, to May 25, 2022.
Interventions: The smartphone-based self-management intervention consisted of an application (app), coach, and website. Over 16 weeks, participants had a coach visit followed by 6 phone calls, and they completed daily and weekly app check-ins. The app provided adaptive feedback and information for developing a personalized wellness plan, the coach provided support, and the website provided summary data and alerts.
Main outcomes and measures: The primary outcome was time to relapse. Secondary outcomes were percentage-time symptomatic, symptom severity, and quality of life.
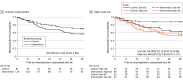
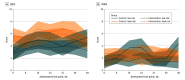
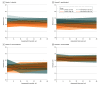
Results: Of the 205 randomized participants (mean [SD] age, 42 [12] years; 125 female individuals [61%]; 5 Asian [2%], 21 Black [10%], 13 Hispanic or Latino [6%], 7 multiracial [3%], 170 White [83%], 2 unknown race [1%]), 81 (40%) were randomly assigned to usual care, and 124 (60%) were randomly assigned to usual care plus the smartphone intervention. This clinical trial did not detect a reduction in relapse risk for the smartphone intervention (hazard ratio [HR], 0.65; 95% CI, 0.39-1.09; log-rank P = .08). However, decreased relapse was observed for low-risk individuals (HR, 0.32; 95% CI, 0.12-0.88; log-rank P = .02) but not high-risk individuals (HR, 0.86; 95% CI, 0.47-1.57; log-rank P = .62). Reduced manic symptom severity was observed for low-risk individuals (mean [SE] difference, -1.4 [0.4]; P = .001) but not for high-risk individuals (mean [SE] difference, 0 [0.3]; P = .95). The smartphone-based self-management intervention decreased depressive symptom severity (mean [SE] difference, -0.80 [0.34]; P = .02) and improved relational quality of life (mean [SE] difference, 1.03 [0.45]; P = .02) but did not decrease percentage-time symptomatic (mean [SE] difference, -5.6 [4.3]; P = .20).
Conclusions and relevance: This randomized clinical trial of a smartphone-based self-management intervention did not detect a significant improvement in the primary outcome of time to relapse. However, a significant decrease in relapse risk was observed for individuals in asymptomatic recovery. In addition, the intervention decreased depressive symptom severity and improved relational quality of life. These findings warrant further work to optimize the smartphone intervention and confirm that the intervention decreases relapse risk for individuals in asymptomatic recovery.
Trial registration: ClinicalTrials.gov Identifier: NCT03088462.
Conflict of interest statement
Figures
Similar articles
-
A Smartphone-Based Self-management Intervention for Individuals With Bipolar Disorder (LiveWell): Empirical and Theoretical Framework, Intervention Design, and Study Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2022 Feb 21;11(2):e30710. doi: 10.2196/30710. JMIR Res Protoc. 2022. PMID: 35188473 Free PMC article.
-
A Smartphone-Based Self-management Intervention for Bipolar Disorder (LiveWell): User-Centered Development Approach.JMIR Ment Health. 2021 Apr 12;8(4):e20424. doi: 10.2196/20424. JMIR Ment Health. 2021. PMID: 33843607 Free PMC article.
-
Effect of an Artificial Intelligence-Based Self-Management App on Musculoskeletal Health in Patients With Neck and/or Low Back Pain Referred to Specialist Care: A Randomized Clinical Trial.JAMA Netw Open. 2023 Jun 1;6(6):e2320400. doi: 10.1001/jamanetworkopen.2023.20400. JAMA Netw Open. 2023. PMID: 37368401 Free PMC article. Clinical Trial.
-
Effect of a Coordinated Community and Chronic Care Model Team Intervention vs Usual Care on Systolic Blood Pressure in Patients With Stroke or Transient Ischemic Attack: The SUCCEED Randomized Clinical Trial.JAMA Netw Open. 2021 Feb 1;4(2):e2036227. doi: 10.1001/jamanetworkopen.2020.36227. JAMA Netw Open. 2021. PMID: 33587132 Free PMC article. Clinical Trial.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
Cited by
-
LiveWell, a smartphone-based self-management intervention for bipolar disorder: Intervention participation and usability analysis.J Affect Disord. 2024 Apr 1;350:926-936. doi: 10.1016/j.jad.2024.01.099. Epub 2024 Jan 19. J Affect Disord. 2024. PMID: 38246280 Free PMC article. Clinical Trial.
-
Audio-Based Care for Managing Mental Health and Substance Use Disorders in Adults: A Systematic Review.Med Care. 2025 Feb 1;63(2):134-151. doi: 10.1097/MLR.0000000000002098. Epub 2025 Jan 9. Med Care. 2025. PMID: 39791847 Free PMC article.
-
Ecological momentary interventions for bipolar disorder: a systematic review and meta-analysis.Soc Psychiatry Psychiatr Epidemiol. 2025 Feb 13. doi: 10.1007/s00127-025-02845-z. Online ahead of print. Soc Psychiatry Psychiatr Epidemiol. 2025. PMID: 39948199 Review.
-
A Brief Video-Based Intervention to Improve Digital Health Literacy for Individuals With Bipolar Disorder: Intervention Development and Results of a Single-Arm Quantitative Pilot Study.J Particip Med. 2025 May 9;17:e59806. doi: 10.2196/59806. J Particip Med. 2025. PMID: 40344658 Free PMC article.
-
Additive effects of adjunctive app-based interventions for mental disorders - A systematic review and meta-analysis of randomised controlled trials.Internet Interv. 2023 Dec 18;35:100703. doi: 10.1016/j.invent.2023.100703. eCollection 2024 Mar. Internet Interv. 2023. PMID: 38225971 Free PMC article. Review.
References
-
- American Psychiatric Association . DSM-5: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013.
-
- Vieta E, Berk M, Schulze TG, et al. . Bipolar disorders. Nat Rev Dis Primers. 2018;4:18008. - PubMed
-
- Keck PE Jr, McElroy SL, Strakowski SM, et al. . 12-month outcome of patients with bipolar disorder following hospitalization for a manic or mixed episode. Am J Psychiatry. 1998;155(5):646-652. - PubMed
-
- Judd LL, Akiskal HS, Schettler PJ, et al. . The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537. - PubMed
-
- Judd LL, Akiskal HS, Schettler PJ, et al. . Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005;62(12):1322-1330. - PubMed

