Significance of two transmembrane ion gradients for human erythrocyte volume stabilization
- PMID: 36542609
- PMCID: PMC9770400
- DOI: 10.1371/journal.pone.0272675
Significance of two transmembrane ion gradients for human erythrocyte volume stabilization
Abstract
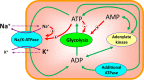
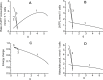
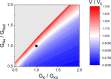
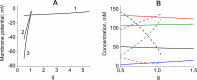
Functional effectiveness of erythrocytes depends on their high deformability that allows them to pass through narrow tissue capillaries. The erythrocytes can deform easily due to discoid shape provided by the stabilization of an optimal cell volume at a given cell surface area. We used mathematical simulation to study the role of transport Na/K-ATPase and transmembrane Na+ and K+ gradients in human erythrocyte volume stabilization at non-selective increase in cell membrane permeability to cations. The model included Na/K-ATPase activated by intracellular Na+, Na+ and K+ transmembrane gradients, and took into account contribution of glycolytic metabolites and adenine nucleotides to cytoplasm osmotic pressure. We found that this model provides the best stabilization of the erythrocyte volume at non-selective increase in the permeability of the cell membrane, which can be caused by an oxidation of the membrane components or mechanical stress during circulation. The volume of the erythrocyte deviates from the optimal value by no more than 10% with a change in the non-selective permeability of the cell membrane to cations from 50 to 200% of the normal value. If only one transmembrane ion gradient is present (Na+), the cell loses the ability to stabilize volume and even small changes in membrane permeability cause dramatic changes in the cell volume. Our results reveal that the presence of two oppositely directed transmembrane ion gradients is fundamentally important for robust stabilization of cellular volume in human erythrocytes.
Copyright: © 2022 Ataullakhanov et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

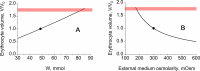
References
-
- Lang F, Foller M, editors. Erythrocytes: Physiology and pathophysiology. London: Imperial College Press; 2012.
-
- Ataullakhanov FI, Vitvitskiĭ VM, Lisovskaia IL, Tuzhilova EG. [Analysis of geometric parameters and mechanical properties of erythrocytes by filtration through nuclear membrane filters. I. A mathematical model] (in Russian). Biofizika. 39: 672–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

