Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study
- PMID: 36542826
- PMCID: PMC10646768
- DOI: 10.1182/blood.2022018730
Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study
Abstract
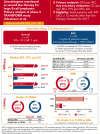
This global phase 3 study compared lisocabtagene maraleucel (liso-cel) with a standard of care (SOC) as second-line therapy for primary refractory or early relapsed (≤12 months) large B-cell lymphoma (LBCL). Adults eligible for autologous stem cell transplantation (ASCT; N = 184) were randomly assigned in a 1:1 ratio to liso-cel (100 × 106 chimeric antigen receptor-positive T cells) or SOC (3 cycles of platinum-based immunochemotherapy followed by high-dose chemotherapy and ASCT in responders). The primary end point was event-free survival (EFS). In this primary analysis with a 17.5-month median follow-up, median EFS was not reached (NR) for liso-cel vs 2.4 months for SOC. Complete response (CR) rate was 74% for liso-cel vs 43% for SOC (P < .0001) and median progression-free survival (PFS) was NR for liso-cel vs 6.2 months for SOC (hazard ratio [HR] = 0.400; P < .0001). Median overall survival (OS) was NR for liso-cel vs 29.9 months for SOC (HR = 0.724; P = .0987). When adjusted for crossover from SOC to liso-cel, 18-month OS rates were 73% for liso-cel and 54% for SOC (HR = 0.415). Grade 3 cytokine release syndrome and neurological events occurred in 1% and 4% of patients in the liso-cel arm, respectively (no grade 4 or 5 events). These data show significant improvements in EFS, CR rate, and PFS for liso-cel compared with SOC and support liso-cel as a preferred second-line treatment compared with SOC in patients with primary refractory or early relapsed LBCL. This trial was registered at www.clinicaltrials.gov as #NCT03575351.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.S.A. reports consulting fees from Bristol Myers Squibb (BMS), AbbVie, Genentech, Epizyme, BeiGene, Kymera, bluebird bio, Incyte, Kite Pharma, Genmab, Ono Pharmaceutical, Mustang Bio, MorphoSys, Regeneron, Century, AstraZeneca, Eli Lilly, and Janssen and received research funding from BMS and Seattle Genetics. J.A. reports honoraria from BMS. B.G. is a consultant for BMS, Roche, Gilead, and Novartis; reports research funding from Roche; is a part of speaker’s bureau at BMS, Roche, and Novartis; is on the advisory boards of BMS and Roche; and is a current employee of Helios Klinikum Berlin-Buch. S.I. is an equity holder in Karyopharm Therapeutics. S. Mielke reports honoraria from Novartis and Celgene, a Bristol-Myers Squibb Company; travel support from Kite/Gilead; and is on the data safety monitoring boards for Miltenyi Biotec and Immunicum. P.M. reports grants or contracts from AstraZeneca and consultancy fees from GlaxoSmithKline. F.H.-I. is on the advisory boards of Kite; Novartis; Celgene, a Bristol-Myers Squibb Company; BMS; Epizyme; Incyte; and AbbVie. K.I. reports consulting fees from BMS, BeiGene, AstraZeneca, Ono Pharmaceutical, AbbVie, Novartis, and Chugai; honoraria from BMS, AstraZeneca, Ono Pharmaceutical, AbbVie, Novartis, Chugai, MSD, Eisai, Janssen, Kyowa Kirin, Daiichi Sankyo, Eli Lilly, SymBio Pharmaceuticals Limited, and Takeda; and research funding from BeiGene, AstraZeneca, Ono Pharmaceutical, AbbVie, Chugai, MSD, Eisai, Incyte, Janssen, Yakult Pharmaceutical Industry Co, Ltd, Daiichi Sankyo, Loxo Oncology, and Genmab. F.M. reports consulting fees from Roche, Gilead, and AbbVie and membership on an entity’s board of directors or advisory committees for Roche, Gilead, Novartis, BMS, AbbVie, Genmab, Miltenyi Biotec, Allogene Therapeutics, AstraZeneca, and Janssen. M.L. reports consulting fees from AbbVie, Acrotech Biopharma, ADC Therapeutics, Astellas, AstraZeneca, BMS, Daiichi Sankyo, EUSA Pharma, Fate Therapeutics, Genentech, Genmab, Janssen/Pharmacyclics, Kite, MorphoSys, Nurix Therapeutics, Pharmacyclics, Seattle Genetics, and TG Therapeutics and research funding from BMS and Curis. A.C. and A.P. are current employees of Celgene, a Bristol-Myers Squibb Company and current equity holders in BMS. S. Montheard is a current employee of Celgene, a Bristol-Myers Squibb Company and a current equity holder in BMS, Novartis Pharma AG, and Alcon. K.O. is a current employee of BMS and current equity holder in BMS. M.K. reports consulting fees from TG Therapeutics, Genentech, AbbVie, AstraZeneca, Adaptive Technologies, ADC Therapeutics, BeiGene, and ImpactBio; reports honoraria from Seagen; and reports research funding and/or data monitoring committee from TG Therapeutics, Genentech, Novartis, and Celgene, a Bristol-Myers Squibb Company. The remaining authors declare no competing financial interests.
Figures


References
-
- Gandhi S, Kallab AM, Hernandez-Ilizaliturri FJ. Diffuse large B-cell lymphoma (DLBCL) guidelines. https://emedicine.medscape.com/article/202969-guidelines Updated 18 November 2022.
-
- Tilly H, Gomes da Silva M, Vitolo U, et al. ESMO Guidelines Committee Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v116–125. - PubMed
-
- Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51(1):51–57. - PubMed
-
- Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32(31):3490–3496. - PubMed
-
- van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35(5):544–551. - PubMed

