Targeting of colorectal cancer organoids with zoledronic acid conjugated to the anti-EGFR antibody cetuximab
- PMID: 36543375
- PMCID: PMC9772689
- DOI: 10.1136/jitc-2022-005660
Targeting of colorectal cancer organoids with zoledronic acid conjugated to the anti-EGFR antibody cetuximab
Abstract
Background: Antibody-drug conjugates (ADC) are essential therapeutic options to treat solid and hematological cancers. The anti-epidermal growth factor-receptor (EGFR) antibody cetuximab (Cet) is used for the therapy of colorectal carcinoma (CRC). Anti-CRC Vδ2 cytolytic T lymphocytes can be elicited by the priming of tumor cells with the aminobisphosphonate zoledronic acid (ZA) and consequent presentation of isopentenyl pyrophosphates through butyrophilin (BTN) family members such as BTN3A1 and BTN2A1. A major drawback that impairs the targeting of ZA to CRC is the bone tropism of aminobisphosphonates.
Methods: The phosphoric group of ZA was linked to free amino groups of Cet in the presence of imidazole following the labeling of phosphoric groups of DNA to amino groups of proteins. The generation of Cet-ZA ADC was confirmed by matrix assisted laser desorption ionization mass spectrometry and inductively coupled plasma-mass spectrometry analysis. Thirteen CRC organoids were obtained with a chemically defined serum-free medium in Geltrex domes. Proliferation and activation of cytolytic activity against CRC organoids by Vδ2 T cells was detected with flow cytometry, crystal violet and cytotoxic probe assays and image analysis. Immunohistochemistry and quantification of BTN3A1 or BTN2A1 expression and the number of tumor infiltrating Vδ2 T cells in CRC were performed by automatic immunostaining, whole slide scanning and computerized analysis of digital pathology imaging.
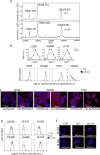
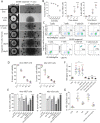
Results: The novel ADC Cet-ZA was generated with a drug antibody ratio of 4.3 and displayed a reactivity similar to the unconjugated antibody. More importantly, patient-derived CRC organoids, or CRC tumor cell suspensions, could trigger the expansion of Vδ2 T cells from peripheral blood and tumor infiltrating lymphocytes when primed with Cet-ZA. Furthermore, Cet-ZA triggered Vδ2 T cell-mediated killing of CRC organoids. The expression of BTN3A1 and BTN2A1 was detected not only in CRC organoids but also in CRC specimens, together with a considerable amount of tumor infiltrating Vδ2 T cells.
Conclusions: These findings are proof of concept that the Cet-ZA ADC can be used to target specifically CRC organoids and may suggest a new experimental approach to deliver aminobisphosphonates to EGFR+ solid tumors.
Keywords: Antibodies, Neoplasm; Cytotoxicity, Immunologic; Gastrointestinal Neoplasms; Lymphocyte Activation.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
Priming of Colorectal Tumor-Associated Fibroblasts with Zoledronic Acid Conjugated to the Anti-Epidermal Growth Factor Receptor Antibody Cetuximab Elicits Anti-Tumor Vδ2 T Lymphocytes.Cancers (Basel). 2023 Jan 18;15(3):610. doi: 10.3390/cancers15030610. Cancers (Basel). 2023. PMID: 36765569 Free PMC article.
-
EGFR-Targeted Antibody-Drug Conjugate to Different Aminobisphosphonates: Direct and Indirect Antitumor Effects on Colorectal Carcinoma Cells.Cancers (Basel). 2024 Mar 22;16(7):1256. doi: 10.3390/cancers16071256. Cancers (Basel). 2024. PMID: 38610932 Free PMC article.
-
Antibody-Drug Conjugate Made of Zoledronic Acid and the Anti-CD30 Brentuximab-Vedotin Exert Anti-Lymphoma and Immunostimulating Effects.Cells. 2024 May 17;13(10):862. doi: 10.3390/cells13100862. Cells. 2024. PMID: 38786084 Free PMC article.
-
ABCA1, apoA-I, and BTN3A1: A Legitimate Ménage à Trois in Dendritic Cells.Front Immunol. 2018 Jun 8;9:1246. doi: 10.3389/fimmu.2018.01246. eCollection 2018. Front Immunol. 2018. PMID: 29937767 Free PMC article. Review.
-
Butyrophilins: γδ T Cell Receptor Ligands, Immunomodulators and More.Front Immunol. 2022 Mar 17;13:876493. doi: 10.3389/fimmu.2022.876493. eCollection 2022. Front Immunol. 2022. PMID: 35371078 Free PMC article. Review.
Cited by
-
Epidermal Growth Factor Receptor Targeting in Colorectal Carcinoma: Antibodies and Patient-Derived Organoids as a Smart Model to Study Therapy Resistance.Int J Mol Sci. 2024 Jun 28;25(13):7131. doi: 10.3390/ijms25137131. Int J Mol Sci. 2024. PMID: 39000238 Free PMC article. Review.
-
Progress of research on γδ T cells in colorectal cancer (Review).Oncol Rep. 2024 Dec;52(6):160. doi: 10.3892/or.2024.8819. Epub 2024 Oct 4. Oncol Rep. 2024. PMID: 39364743 Free PMC article. Review.
-
Signaling pathways in colorectal cancer implications for the target therapies.Mol Biomed. 2024 Jun 7;5(1):21. doi: 10.1186/s43556-024-00178-y. Mol Biomed. 2024. PMID: 38844562 Free PMC article. Review.
-
Medulloblastoma: biology and immunotherapy.Front Immunol. 2025 Jul 3;16:1602930. doi: 10.3389/fimmu.2025.1602930. eCollection 2025. Front Immunol. 2025. PMID: 40677711 Free PMC article. Review.
-
Novel SPEA Superantigen Peptide Agonists and Peptide Agonist-TGFαL3 Conjugate. In Vitro Study of Their Growth-Inhibitory Effects for Targeted Cancer Immunotherapy.Int J Mol Sci. 2023 Jun 22;24(13):10507. doi: 10.3390/ijms241310507. Int J Mol Sci. 2023. PMID: 37445686 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
