Modeling resistance of colorectal peritoneal metastases to immune checkpoint blockade in humanized mice
- PMID: 36543378
- PMCID: PMC9772695
- DOI: 10.1136/jitc-2022-005345
Modeling resistance of colorectal peritoneal metastases to immune checkpoint blockade in humanized mice
Abstract
Background: The immunogenic nature of metastatic colorectal cancer (CRC) with high microsatellite instability (MSI-H) underlies their responsiveness to immune checkpoint blockade (ICB). However, resistance to ICB is commonly observed, and is associated with the presence of peritoneal-metastases and ascites formation. The mechanisms underlying this site-specific benefit of ICB are unknown.
Methods: We created a novel model for spontaneous multiorgan metastasis in MSI-H CRC tumors by transplanting patient-derived organoids (PDO) into the cecum of humanized mice. Anti-programmed cell death protein-1 (PD-1) and anti-cytotoxic T-lymphocytes-associated protein 4 (CTLA-4) ICB treatment effects were analyzed in relation to the immune context of primary tumors, liver metastases, and peritoneal metastases. Immune profiling was performed by immunohistochemistry, flow cytometry and single-cell RNA sequencing. The role of B cells was assessed by antibody-mediated depletion. Immunosuppressive cytokine levels (interleukin (IL)-10, transforming growth factor (TGF)b1, TGFb2, TGFb3) were determined in ascites and serum samples by ELISA.
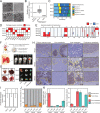
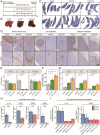
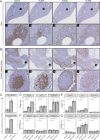
Results: PDO-initiated primary tumors spontaneously metastasized to the liver and the peritoneum. Peritoneal-metastasis formation was accompanied by the accumulation of ascites. ICB completely cleared liver metastases and reduced primary tumor mass but had no effect on peritoneal metastases. This mimics clinical observations. After therapy discontinuation, primary tumor masses progressively decreased, but peritoneal metastases displayed unabated growth. Therapy efficacy correlated with the formation of tertiary lymphoid structures (TLS)-containing B cells and juxtaposed T cells-and with expression of an interferon-γ signature together with the B cell chemoattractant CXCL13. B cell depletion prevented liver-metastasis clearance by anti-CTLA-4 treatment. Peritoneal metastases were devoid of B cells and TLS, while the T cells in these lesions displayed a dysfunctional phenotype. Ascites samples from patients with cancer with peritoneal metastases and from the mouse model contained significantly higher levels of IL-10, TGFb1, TGFb2 and TGFb3 than serum samples.
Conclusions: By combining organoid and humanized mouse technologies, we present a novel model for spontaneous multiorgan metastasis by MSI-H CRC, in which the clinically observed organ site-dependent benefit of ICB is recapitulated. Moreover, we provide empirical evidence for a critical role for B cells in the generation of site-dependent antitumor immunity following anti-CTLA-4 treatment. High levels of immunosuppressive cytokines in ascites may underlie the observed resistance of peritoneal metastases to ICB.
Keywords: B-lymphocytes; CTLA-4 antigen; immunotherapy; programmed cell death 1 receptor; tumor microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: EK, BAH, JV, AV, MC, SvH, IHMBR, CJAP, JL, HS, and OK have no conflicts of interest to disclose. SFB is the inventor on patents related to the Organoid Technology. SFB is employed by the Foundation Hubrecht Organoid Technology. MK reports having an advisory role for Nordic Farma, Merck-Serono, Pierre Fabre, Servier, and MSD and institutional scientific grants from Bayer, Bristol Myers Squibb, Merck, Nordic Farma, Pierre Fabre Roche, Servier, and Sirtex. JMLR reports having an advisory role for Merck-Serono, Pierre Fabre, Servier, MSD, Bayer, and Bristol Myers Squibb and institutional scientific grants from Bristol Myers Squibb, Pierre Fabre, Servier, and Hubrecht Organoid Technology. All grants were unrelated to the study and were paid to the individual’s institution.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
