Hematopoietic Stem Cell Transplantation in People With Active Secondary Progressive Multiple Sclerosis
- PMID: 36543569
- PMCID: PMC10074454
- DOI: 10.1212/WNL.0000000000206750
Hematopoietic Stem Cell Transplantation in People With Active Secondary Progressive Multiple Sclerosis
Abstract
Background and objectives: Uncontrolled evidence suggests that autologous hematopoietic stem cell transplantation (AHSCT) can be effective in people with active secondary progressive multiple sclerosis (SPMS). In this study, we compared the effect of AHSCT with that of other anti-inflammatory disease-modifying therapies (DMTs) on long-term disability worsening in active SPMS.
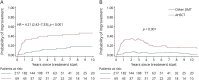
Methods: We collected data from the Italian Bone Marrow Transplantation Study Group and the Italian Multiple Sclerosis Register. Patients were considered eligible if treatment had been started after the diagnosis of SPMS. Disability worsening was assessed by the cumulative proportion of patients with a 6-month confirmed disability progression (CDP) according to the Expanded Disability Status Scale (EDSS) score. Key secondary endpoints were the EDSS time trend after treatment start and the prevalence of disability improvement over time. Time to first CDP was assessed by means of proportional hazard Cox regression models. A linear mixed model with a time × treatment group interaction was used to assess the longitudinal EDSS time trends. Prevalence of improvement was estimated using a modified Kaplan-Meier estimator and compared between groups by bootstrapping the area under the curve.
Results: Seventy-nine AHSCT-treated patients and 1975 patients treated with other DMTs (beta interferons, azathioprine, glatiramer-acetate, mitoxantrone, fingolimod, natalizumab, methotrexate, teriflunomide, cyclophosphamide, dimethyl fumarate, and alemtuzumab) were matched to reduce treatment selection bias using propensity score and overlap weighting approaches. Time to first CDP was significantly longer in transplanted patients (hazard ratio [HR] = 0.50; 95% CI = 0.31-0.81; p = 0.005), with 61.7% of transplanted patients free from CPD at 5 years. Accordingly, EDSS time trend over 10 years was higher in patients treated with other DMTs than in AHSCT-treated patients (+0.157 EDSS points per year compared with -0.013 EDSS points per year; interaction p < 0.001). Patients who underwent AHSCT were more likely to experience a sustained disability improvement: 34.7% of patients maintained an improvement (a lower EDSS than baseline) 3 years after transplant vs 4.6% of patients treated by other DMTs (p < 0.001).
Discussion: The use of AHSCT in people with active SPMS is associated with a slowing of disability progression and a higher likelihood of disability improvement compared with standard immunotherapy.
Classification of evidence: This study provides Class III evidence that autologous hematopoietic stem cell transplants prolonged the time to CDP compared with other DMTs.
© 2022 American Academy of Neurology.
Conflict of interest statement
G. Boffa was supported by a research fellowship FISM - Fondazione Italiana Sclerosi Multipla 2019/BR/016 and financed or cofinanced with the “5 per mille” public funding. A. Signori has nothing to disclose. L. Massacesi received educational grants and/or research funds from Fondazione Cassa di Risparmio di Firenze, Biogen, Merck-Serono, Genzyme, and Roche and received honoraria or consultation fees from Biogen, Roche, Mylan, Merck-Serono, Genzyme, and Novartis. A. Mariottini reports nonfinancial support from Biogen idec, Sanofi Genzyme, Novartis, Teva, and Roche and personal fees from Merck Serono. E. Sbragia has nothing to disclose. S. Cottone received grants and honoraria from Roche, Sanofi, Merck, Biogen, and Novartis. M.P. Amato has served on Scientific Advisory Boards for Biogen, Novartis, Roche, Merck, Sanofi Genzyme, and Teva; received speaker honoraria from Biogen, Merck, Sanofi Genzyme, Roche, Novartis, and Teva; and received research grants for her Institution from Biogen, Merck, Sanofi Genzyme, Novartis, and Roche. C. Gasperini reports fees as invited speaker or travel expenses for attending meeting from Biogen, Merck‐Serono, Teva, Mylan, Sanofi, Novartis, and Genzyme. LP: consulting fees from Biogen, Novartis, and Roche; speaker honoraria from Biogen, Genzyme, Merck Serono, Mylan, Novartis, and Teva; travel grants from Biogen, Genzyme, Novartis, and Teva; and research grants from the Italian MS Society (Associazione Italiana Sclerosi Multipla) and Genzyme. L. Moiola has received speaker's honoraria from the following companies: Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and TEVA. S. Meletti has nothing to disclose. V. Brescia Morra has received funding for research support and speaker honoraria from Novartis, Roche, Biogen, Teva, Almirall, Sanofi-Genzyme, Merk, Bayer, and Mylan. M. Trojano has served on scientific AB for Biogen, Novartis, Roche, Merck, and Genzyme; received speaker honoraria from Biogen, Roche, Sanofi, Merck, Genzyme, and Novartis; and received research grants for her Institution from Biogen, Merck, and Novartis. G. Salemi received grants and honoraria from Roche, Sanofi, Merck, Biogen, and Novartis. F. Patti reports consulting fees from Alexion, Almirall, Bayer, Biogen, Calgene, Merck, Myalin, Novartis, Roche, Sanofi, and TEVA and research grants from Biogen and Merck. M. Filippi is Editor-in-Chief of the Journal of Neurology and Associate Editor of Human Brain Mapping, received compensation for consulting services and/or speaking activities from Almiral, Alexion, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries, and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). G. De Luca reports travel grants and/or speaker honoraria from Merck-Serono, Roche, Sanofi Genzyme, and Biogen. G. Lus has nothing to disclose. M. Zaffaroni received travel support and fees for lecturing or participating in advisory boards from Almirall, Biogen, Merck, Novartis, and Sanofi-Genzyme. P. Sola received travel grants from Teva, Roche, Sanofi, Merck, Biogen, Bristol, and Novartis and fees for consultation from Merck, Biogen, and Sanofi Genzyme. A. Conte received grants from Roche and fees for consultation from Sanofi Genzyme, Merck, Biogen, Almirall, and Novartis. R. Nistri has nothing to disclose. U. Aguglia has nothing to disclose. F. Granella received research grants from Biogen and Roche and fees for consultation from Biogen, Sanofi, Merck Serono, Novartis, and Roche. S. Galgani received fees as invited speaker or travel expenses for attending meeting from Biogen, Merck‐Serono, Teva, Almirall, Sanofi‐Aventis, Novartis, and Genzyme. L.M. Caniatti has nothing to disclose. A. Lugaresi has served as a Biogen, Merck Serono, Novartis, Roche, Sanofi/Genzyme, and Teva Advisory Board Member. She received congress and travel/accommodation expense compensations or speaker honoraria from Biogen, Merck, Mylan, Novartis, Sanofi/Genzyme, Teva, and Fondazione Italiana Sclerosi Multipla (FISM). Her institutions received research grants from Novartis. S. Romano reports fees as speaker and travel expense refunds from Biogen, Novartis, and Roche. P. Iaffaldano has served on scientific advisory boards for Biogen Idec, Bayer, Teva, Roche, Merck Serono, Novartis, and Genzyme and has received funding for travel and/or speaker honoraria from Sanofi Aventis, Genzyme, Biogen Idec, Teva, Merck Serono, and Novartis. R. Saccardi has nothing to disclose. E. Angelucci is DMC member for Celgene and VERTEX-CRISPR-CAS9, Adv board for Novartis, BlueBirdBio, and Gilead. G.L. Mancardi received support from Biogen Idec (honoraria for lecturing, travel expenses for attending meetings, and financial support for research), Genzyme (honorarium for lecturing), Merck Serono, Novartis, Teva (financial support for research), and Sanofi Aventis (honorarium for speaking). M.P. Sormani received consulting fees from Biogen Idec, Merck Serono, Teva, Genzyme, Roche, Novartis, GeNeuro, and Medday. M. Inglese received grants NIH, NMSS, and FISM and received fees for consultation from Roche, Genzyme, Merck, Biogen, and Novartis. Go to
Figures


References
-
- Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 2018;135(4):511-528. doi: 10.1007/s00401-018-1818-y - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
