Soluble components from mesenchymal stromal cell processing exert anti-inflammatory effects and facilitate ischemic muscle regeneration
- PMID: 36543717
- PMCID: PMC10006307
- DOI: 10.1016/j.jcyt.2022.11.010
Soluble components from mesenchymal stromal cell processing exert anti-inflammatory effects and facilitate ischemic muscle regeneration
Abstract
Background aims: Skeletal muscle regeneration after severe damage is reliant on local stem cell proliferation and differentiation, processes that are tightly regulated by macrophages. Peripheral artery disease is a globally prevalent cardiovascular disease affecting millions of people. Progression of the disease leads to intermittent claudication, subsequent critical limb ischemia and muscle injury. Tissue-derived and ex vivo-expanded mesenchymal stromal cells (MSCs) for skeletal muscle regeneration have been studied, but pre-clinical and clinical results have not been consistent. As a result, the potential therapeutic efficacy and associated repair mechanisms of MSCs remain unclear. Numerous studies have demonstrated the vulnerability of delivered MSCs, with a precipitous drop in cell viability upon transplantation. This has prompted investigation into the therapeutic benefit of apoptotic cells, microvesicles, exosomes and soluble signals that are released upon cell death.
Methods: In this study, we characterized various components produced by MSCs after cell death induction under different conditions. We discovered anti-inflammatory and pro-regenerative effects produced by cell components following a freeze and thaw (F&T) process on macrophage polarization in vitro. We further investigated the underlying mechanisms of macrophage polarization by those components resulting from severe cell death induction.
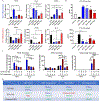
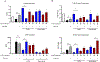
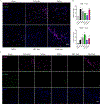
Results: We found potent therapeutic effects from F&T-induced cell debris are dependent on the externalization of phosphatidylserine on the plasma membrane. In contrast, effects from the supernatant of F&T-induced cell death primarily depends on the released protein content. We then applied the F&T-induced cell supernatant to an animal model of peripheral artery disease to treat muscle injury caused by severe ischemia. Treatment with the F&T supernatant but not the vulnerable MSCs resulted in significantly improved recovery of muscle function, blood flow and morphology and inflammation resolution in the affected muscles 2 weeks after injury.
Conclusions: This study validates the therapeutic potential of F&T-induced supernatant obviating the need for a viable population from vulnerable MSCs to treat injury, thus providing a roadmap for cell-free therapeutic approaches for tissue regeneration.
Keywords: cell death; immunomodulation; macrophage polarization; mesenchymal stromal cells; muscle regeneration; stem cell therapy.
Copyright © 2022 International Society for Cell & Gene Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no commercial, proprietary or financial interest in the products or companies described in this article.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

