Sourcing thermotolerant poly(ethylene terephthalate) hydrolase scaffolds from natural diversity
- PMID: 36543766
- PMCID: PMC9772341
- DOI: 10.1038/s41467-022-35237-x
Sourcing thermotolerant poly(ethylene terephthalate) hydrolase scaffolds from natural diversity
Abstract
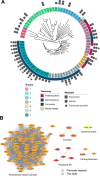
Enzymatic deconstruction of poly(ethylene terephthalate) (PET) is under intense investigation, given the ability of hydrolase enzymes to depolymerize PET to its constituent monomers near the polymer glass transition temperature. To date, reported PET hydrolases have been sourced from a relatively narrow sequence space. Here, we identify additional PET-active biocatalysts from natural diversity by using bioinformatics and machine learning to mine 74 putative thermotolerant PET hydrolases. We successfully express, purify, and assay 51 enzymes from seven distinct phylogenetic groups; observing PET hydrolysis activity on amorphous PET film from 37 enzymes in reactions spanning pH from 4.5-9.0 and temperatures from 30-70 °C. We conduct PET hydrolysis time-course reactions with the best-performing enzymes, where we observe differences in substrate selectivity as function of PET morphology. We employed X-ray crystallography and AlphaFold to examine the enzyme architectures of all 74 candidates, revealing protein folds and accessory domains not previously associated with PET deconstruction. Overall, this study expands the number and diversity of thermotolerant scaffolds for enzymatic PET deconstruction.
© 2022. The Author(s).
Conflict of interest statement
U.S. Patent Application No.: 63/297,529 was submitted by the National Renewable Energy Laboratory (NREL), operated by Alliance for Sustainable Energy, LLC. The inventors include E.E., J.E.G., C.M.P., G.T.B., and J.E.M. The other authors declare no competing interests.
Figures




References
-
- Sinha V, Patel MR, Patel JV. PET waste management by chemical recycling: a review. J. Polym. Environ. 2010;18:8–25. doi: 10.1007/s10924-008-0106-7. - DOI
-
- Rahimi A, García JM. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017;1:1–11. doi: 10.1038/s41570-017-0046. - DOI
-
- Ellis LD, et al. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021;4:539–556. doi: 10.1038/s41929-021-00648-4. - DOI
-
- Martín, A. J., Mondelli, C., Jaydev, S. D. & Pérez-Ramírez, J. Catalytic processing of plastic waste on the rise. Chem 7, 1487–1533 (2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

