Calcium-permeable channelrhodopsins for the photocontrol of calcium signalling
- PMID: 36543773
- PMCID: PMC9772239
- DOI: 10.1038/s41467-022-35373-4
Calcium-permeable channelrhodopsins for the photocontrol of calcium signalling
Erratum in
-
Author Correction: Calcium-permeable channelrhodopsins for the photocontrol of calcium signalling.Nat Commun. 2024 May 10;15(1):3958. doi: 10.1038/s41467-024-48356-4. Nat Commun. 2024. PMID: 38730225 Free PMC article. No abstract available.
Abstract
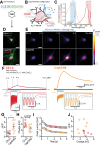
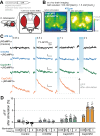
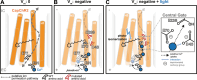
Channelrhodopsins are light-gated ion channels used to control excitability of designated cells in large networks with high spatiotemporal resolution. While ChRs selective for H+, Na+, K+ and anions have been discovered or engineered, Ca2+-selective ChRs have not been reported to date. Here, we analyse ChRs and mutant derivatives with regard to their Ca2+ permeability and improve their Ca2+ affinity by targeted mutagenesis at the central selectivity filter. The engineered channels, termed CapChR1 and CapChR2 for calcium-permeable channelrhodopsins, exhibit reduced sodium and proton conductance in connection with strongly improved Ca2+ permeation at negative voltage and low extracellular Ca2+ concentrations. In cultured cells and neurons, CapChR2 reliably increases intracellular Ca2+ concentrations. Moreover, CapChR2 can robustly trigger Ca2+ signalling in hippocampal neurons. When expressed together with genetically encoded Ca2+ indicators in Drosophila melanogaster mushroom body output neurons, CapChRs mediate light-evoked Ca2+ entry in brain explants.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

