Alterations of the vaginal microbiome in healthy pregnant women positive for group B Streptococcus colonization during the third trimester
- PMID: 36544085
- PMCID: PMC9769055
- DOI: 10.1186/s12866-022-02730-8
Alterations of the vaginal microbiome in healthy pregnant women positive for group B Streptococcus colonization during the third trimester
Abstract
Background: Streptococcus agalactiae or group B Streptococcus (GBS) asymptomatically colonizes the genitourinary tracts of up to 30% of pregnant women. Globally, GBS is an important cause of neonatal morbidity and mortality. GBS has recently been linked to adverse pregnancy outcomes. The potential interactions between GBS and the vaginal microbiome composition remain poorly understood. In addition, little is known about the vaginal microbiota of pregnant Egyptian women.
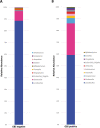
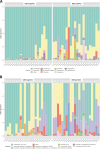
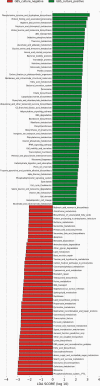
Results: Using V3-V4 16S rRNA next-generation sequencing, we examined the vaginal microbiome in GBS culture-positive pregnant women (22) and GBS culture-negative pregnant women (22) during the third trimester in Ismailia, Egypt. According to the alpha-diversity indices, the vaginal microbiome of pregnant GBS culture-positive women was significantly more diverse and less homogenous. The composition of the vaginal microbiome differed significantly based on beta-diversity between GBS culture-positive and culture-negative women. The phylum Firmicutes and the family Lactobacillaceae were significantly more abundant in GBS-negative colonizers. In contrast, the phyla Actinobacteria, Tenericutes, and Proteobacteria and the families Bifidobacteriaceae, Mycoplasmataceae, Streptococcaceae, Corynebacteriaceae, Staphylococcaceae, and Peptostreptococcaceae were significantly more abundant in GBS culture-positive colonizers. On the genus and species levels, Lactobacillus was the only genus detected with significantly higher relative abundance in GBS culture-negative status (88%), and L. iners was the significantly most abundant species. Conversely, GBS-positive carriers exhibited a significant decrease in Lactobacillus abundance (56%). In GBS-positive colonizers, the relative abundance of the genera Ureaplasma, Gardnerella, Streptococcus, Corynebacterium, Staphylococcus, and Peptostreptococcus and the species Peptostreptococcus anaerobius was significantly higher. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways related to the metabolism of cofactors and vitamins, phosphatidylinositol signaling system, peroxisome, host immune system pathways, and host endocrine system were exclusively enriched among GBS culture-positive microbial communities. However, lipid metabolism KEGG pathways, nucleotide metabolism, xenobiotics biodegradation and metabolism, genetic information processing pathways associated with translation, replication, and repair, and human diseases (Staphylococcus aureus infection) were exclusively enriched in GBS culture-negative communities.
Conclusions: Understanding how perturbations of the vaginal microbiome contribute to pregnancy complications may result in the development of alternative, targeted prevention strategies to prevent maternal GBS colonization. We hypothesized associations between inferred microbial function and GBS status that would need to be confirmed in larger cohorts.
Keywords: Group B Streptococcus; Healthy; Microbiome; Pregnant; Streptococcus agalactiae; Third trimester; Vagina.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Group B Streptococcus vaginal colonisation throughout pregnancy is associated with decreased Lactobacillus crispatus and increased Lactobacillus iners abundance in the vaginal microbial community.Front Cell Infect Microbiol. 2024 Sep 30;14:1435745. doi: 10.3389/fcimb.2024.1435745. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39403199 Free PMC article.
-
An analysis of the vaginal microbiota in women positive for group B Streptococcus during the third trimester of pregnancy.BMC Microbiol. 2025 Jul 26;25(1):454. doi: 10.1186/s12866-025-04184-0. BMC Microbiol. 2025. PMID: 40713506 Free PMC article.
-
Diversity and composition of vaginal microbiota of pregnant women at risk for transmitting Group B Streptococcus treated with intrapartum penicillin.PLoS One. 2017 Feb 8;12(2):e0169916. doi: 10.1371/journal.pone.0169916. eCollection 2017. PLoS One. 2017. PMID: 28178310 Free PMC article.
-
Vaginal-perianal or vaginal-perineal compared with vaginal-rectal culture-based screening for Group B Streptococci (GBS) colonization during the third trimester of pregnancy: a systematic review and meta-analysis.BMC Pregnancy Childbirth. 2022 Mar 14;22(1):204. doi: 10.1186/s12884-022-04546-w. BMC Pregnancy Childbirth. 2022. PMID: 35287615 Free PMC article.
-
Interspecies Interactions within the Host: the Social Network of Group B Streptococcus.Infect Immun. 2023 Apr 18;91(4):e0044022. doi: 10.1128/iai.00440-22. Epub 2023 Mar 28. Infect Immun. 2023. PMID: 36975791 Free PMC article. Review.
Cited by
-
Maternal group B Streptococcus decreases infant length and alters the early-life microbiome: a prospective cohort study.Ann Med. 2025 Dec;57(1):2442070. doi: 10.1080/07853890.2024.2442070. Epub 2024 Dec 18. Ann Med. 2025. PMID: 39693119 Free PMC article.
-
Vaginal Microbiome: Environmental, Biological, and Racial Influences on Gynecological Health Across the Lifespan.Am J Reprod Immunol. 2024 Dec;92(6):e70026. doi: 10.1111/aji.70026. Am J Reprod Immunol. 2024. PMID: 39670915 Free PMC article. Review.
-
Detection of sexually transmitted infection agents in pregnant women using multiplex polymerase chain reaction method.BMC Pregnancy Childbirth. 2025 Mar 18;25(1):307. doi: 10.1186/s12884-025-07430-5. BMC Pregnancy Childbirth. 2025. PMID: 40102772 Free PMC article.
-
HIV-1 interaction with an O-glycan-specific bacterial lectin enhances virus infectivity and resistance to neutralizing antibodies.iScience. 2024 Jun 27;27(8):110390. doi: 10.1016/j.isci.2024.110390. eCollection 2024 Aug 16. iScience. 2024. PMID: 39108723 Free PMC article.
-
Gestational diabetes as a risk factor for GBS maternal rectovaginal colonization: a systematic review and meta-analysis.BMC Pregnancy Childbirth. 2024 Jul 20;24(1):488. doi: 10.1186/s12884-024-06694-7. BMC Pregnancy Childbirth. 2024. PMID: 39033123 Free PMC article.
References
-
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(Rr-10):1–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

