Lecanemab in patients with early Alzheimer's disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study
- PMID: 36544184
- PMCID: PMC9768996
- DOI: 10.1186/s13195-022-01124-2
Lecanemab in patients with early Alzheimer's disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study
Abstract
Background: Lecanemab, a humanized IgG1 monoclonal antibody that targets soluble aggregated Aβ species (protofibrils), has demonstrated robust brain fibrillar amyloid reduction and slowing of clinical decline in early AD. The objective of this analysis is to report results from study 201 blinded period (core), the open-label extension (OLE), and gap period (between core and OLE) supporting the effectiveness of lecanemab.
Methods: The lecanemab study 201 core was a double-blind, randomized, placebo-controlled study of 856 patients randomized to one of five dose regimens or placebo. An OLE of study 201 was initiated to allow patients to receive open-label lecanemab 10mg/kg biweekly for up to 24 months, with an intervening off-treatment period (gap period) ranging from 9 to 59 months (mean 24 months).
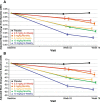
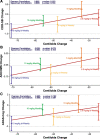
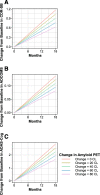
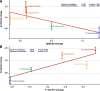
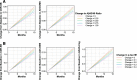
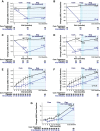
Results: At 12 and 18 months of treatment in the core, lecanemab 10 mg/kg biweekly demonstrated dose-dependent reductions of brain amyloid measured PET and corresponding changes in plasma biomarkers and slowing of cognitive decline. The rates of clinical progression during the gap were similar in lecanemab and placebo subjects, with clinical treatment differences maintained after discontinued dosing over an average of 24 months in the gap period. During the gap, plasma Aβ42/40 ratio and p-tau181 levels began to return towards pre-randomization levels more quickly than amyloid PET. At OLE baseline, treatment differences vs placebo at 18 months in the randomized period were maintained across 3 clinical assessments. In the OLE, lecanemab 10 mg/kg biweekly treatment produced dose-dependent reductions in amyloid PET SUVr, improvements in plasma Aβ42/40 ratio, and reductions in plasma p-tau181.
Conclusions: Lecanemab treatment resulted in significant reduction in amyloid plaques and a slowing of clinical decline. Data indicate that rapid and pronounced amyloid reduction correlates with clinical benefit and potential disease-modifying effects, as well as the potential to use plasma biomarkers to monitor for lecanemab treatment effects.
Trial registration: ClinicalTrials.gov NCT01767311 .
© 2022. The Author(s).
Conflict of interest statement
EM is the Associate Director of the DIAN–TU. He reports serving on a Data Safety Committee for Eli Lilly and Company and Alector. He is scientific consultant for Eisai and Eli Lilly and Company and has received institutional grant support from Eli Lilly and Company, F. Hoffmann-La Roche Ltd. and Janssen. JC has provided consultation to Acadia, Actinogen, Alkahest, AlphaCognition, AriBio, Biogen, Cassava, Cerecin, Cortexyme, Diadem, EIP Pharma, Eisai, eqt, GemVax, Genentech, Green Valley, GAP Innovations, Grifols, Janssen, Karuna, Lilly, Lundbeck, Merck, NervGen, Novo Nordisk, Oligomerix, Optoceutics, Ono, Otsuka, PRODEO, Prothena, ReMYND, Resverlogix, Roche, Sage Therapeutics, Signant Health, Suven, TrueBinding, and Vaxxinity pharmaceutical, assessment, and investment companies. JC is supported by NIGMS grant P20GM109025; NINDS grant U01NS093334; NIA grant R01AG053798; NIA grant P20AG068053; NIA grant P30AG072959; NIA grant R35AG71476; Alzheimer’s Disease Drug Discovery Foundation (ADDF); Ted and Maria Quirk Endowment; and the Joy Chambers-Grundy Endowment. SD, CS, LR, MK, AK, MI, and LK are employees of Eisai. RJB is Director of DIAN–TU and Principal Investigator of DIAN–TU-001. He receives research support from the NIA of the NIH, DIAN–TU trial pharmaceutical partners (Eli Lilly and Company, F. Hoffman-La Roche Ltd and Avid Radiopharmaceuticals), Alzheimer’s Association, GHR Foundation, Anonymous Organization, DIAN–TU Pharma Consortium (active: Biogen, Eisai, Eli Lilly and Company, Janssen, F. Hoffmann-La Roche Ltd/Genentech; previous: AbbVie, Amgen, AstraZeneca, Forum, Mithridion, Novartis, Pfizer, Sanofi, United Neuroscience). He has been an invited speaker and consultant for AC Immune, F. Hoffman-La Roche Ltd and Janssen and a consultant for Amgen and Eisai. The author(s) read and approved the final manuscript
Figures



References
-
- Masters C, Bateman R, Blennow K, et al. Alzheimer’s disease. Nature Reviews. 2015;1:15056. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

