Retinal pigment epithelium extracellular vesicles are potent inducers of age-related macular degeneration disease phenotype in the outer retina
- PMID: 36544284
- PMCID: PMC9772497
- DOI: 10.1002/jev2.12295
Retinal pigment epithelium extracellular vesicles are potent inducers of age-related macular degeneration disease phenotype in the outer retina
Abstract
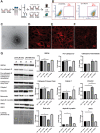
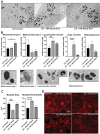
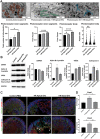
Age-related macular degeneration (AMD) is a leading cause of blindness. Vision loss is caused by the retinal pigment epithelium (RPE) and photoreceptors atrophy and/or retinal and choroidal angiogenesis. Here we use AMD patient-specific RPE cells with the Complement Factor H Y402H high-risk polymorphism to perform a comprehensive analysis of extracellular vesicles (EVs), their cargo and role in disease pathology. We show that AMD RPE is characterised by enhanced polarised EV secretion. Multi-omics analyses demonstrate that AMD RPE EVs carry RNA, proteins and lipids, which mediate key AMD features including oxidative stress, cytoskeletal dysfunction, angiogenesis and drusen accumulation. Moreover, AMD RPE EVs induce amyloid fibril formation, revealing their role in drusen formation. We demonstrate that exposure of control RPE to AMD RPE apical EVs leads to the acquisition of AMD features such as stress vacuoles, cytoskeletal destabilization and abnormalities in the morphology of the nucleus. Retinal organoid treatment with apical AMD RPE EVs leads to disrupted neuroepithelium and the appearance of cytoprotective alpha B crystallin immunopositive cells, with some co-expressing retinal progenitor cell markers Pax6/Vsx2, suggesting injury-induced regenerative pathways activation. These findings indicate that AMD RPE EVs are potent inducers of AMD phenotype in the neighbouring RPE and retinal cells.
Keywords: age-related macular degeneration; complement factor H; extracellular vesicles; human induced pluripotent stem cells; photoreceptors; retina; retinal pigment epithelium.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no relevant conflict of interest.
Figures
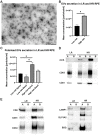
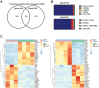
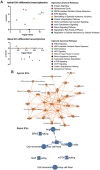

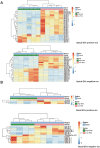

References
-
- Ahmad, I. , Del Debbio, C. B. , Das, A. V. , & Parameswaran, S. (2011). Muller glia: A promising target for therapeutic regeneration. Investigative Ophthalmology & Visual Science, 52(8), 5758–5764. - PubMed
-
- Anderson, D. H. , Talaga, K. C. , Rivest, A. J. , Barron, E. , Hageman, G. S. , & Johnson, L. V. (2004). Characterization of beta amyloid assemblies in drusen: The deposits associated with aging and age‐related macular degeneration. Experimental Eye Research, 78(2), 243–256. - PubMed
-
- Atienzar‐Aroca, S. , Serrano‐Heras, G. , Freire Valls, A. , Ruiz de Almodovar, C. , Muriach, M. , Barcia, J. M. , Garcia‐Verdugo, J. M. , Romero, F. J. , & Sancho‐Pelluz, J. (2018). Role of retinal pigment epithelium‐derived exosomes and autophagy in new blood vessel formation. Journal of Cellular and Molecular Medicine, 22(11), 5244–5256. - PMC - PubMed
-
- Azuma, M. , Fukiage, C. , David, L. L. , & Shearer, T. R. (1997). Activation of calpain in lens: A review and proposed mechanism. Experimental Eye Research, 64(4), 529–538. - PubMed
Publication types
MeSH terms
Grants and funding
- MR/S036695/1/MRC_/Medical Research Council/United Kingdom
- BBSRC BB/T000805/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R013942/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- NC/C016106/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- MR/L009560/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

